Editor: Nina
Latest News on Lipid Nanoparticles for mRNA Delivery
Lipid nanoparticles (LNPs) have gained significant attention as a key vehicle for delivering mRNA-based therapeutics. Most recently, the success of LNP-based mRNA vaccines for COVID-19 has highlighted their potential in the therapeutic landscape. These LNPs are engineered to protect fragile mRNA molecules and facilitate their delivery into cells. The FDA-approved LNP formulations for mRNA vaccines and siRNA therapies have established the clinical utility of this delivery platform, paving the way for their use in other applications like cancer treatment and genetic diseases.
Latest Advancements in Lipid Nanoparticles for mRNA Delivery
Compared to traditional methods, such as viral vectors or electroporation, the latest advancements in LNP technology offer significant improvements in stability, safety, and efficiency. For instance, the LNPs developed for mRNA delivery have evolved to include ionizable lipids, which allow the particles to remain neutral in physiological conditions and become positively charged in acidic environments, facilitating endosomal escape. Moreover, new studies show that the design of LNPs can be further optimized to enhance their delivery to specific cell types, such as T cells for cancer immunotherapy. These innovations overcome many of the limitations of older technologies, including toxicity and low delivery efficiency, making LNPs a promising tool for clinical applications.
Overview of the Papers Discussed
There are four papers, each presenting unique contributions to the development of LNPs for mRNA delivery. The research is being conducted by teams from various universities, including The Ohio State University and Oregon Health & Science University, as well as international collaborations. The diseases targeted include cancers (e.g., acute lymphoblastic leukemia), genetic disorders, and potential applications in prenatal treatments. The key focus of the research is on optimizing the LNP formulations to improve mRNA delivery, with particular attention to their long-term stability, tissue-specific delivery, and minimal toxicity
Paper 1: Ionizable Lipid Nanoparticle-Mediated mRNA Delivery for Human CAR T Cell Engineering

The paper “Ionizable Lipid Nanoparticle-Mediated mRNA Delivery for Human CAR T Cell Engineering” was led by Margaret M. Billingsley, published in Nano Letters.
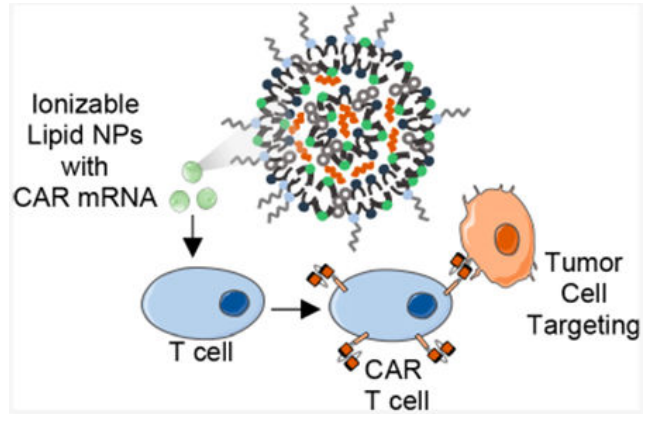
The research addresses the challenges of viral delivery systems in CAR T cell therapies, such as immunogenicity and permanent CAR expression, which can cause severe side effects. The study explores the use of ionizable lipid nanoparticles (LNPs) to deliver mRNA that induces transient CAR expression in T cells, avoiding the risks associated with viral vectors. The key innovation of this study is the development of a library of LNPs, screened for their ability to deliver mRNA to T cells with minimal toxicity. The top-performing formulation, C14-4 LNP, successfully induced CAR expression in T cells without the cytotoxicity typically associated with electroporation. The study used a variety of methods, including microfluidic devices for LNP formulation and luciferase mRNA screening to identify the optimal LNP formulation. The key findings show that LNPs can efficiently deliver mRNA to human T cells, producing functional CAR T cells with potent cancer-killing activity in leukemia models. This research is significant because it demonstrates a safer, less expensive alternative to viral vector-based CAR T cell engineering, which could make the therapy more accessible and reduce patient risk.
Paper 2: Long-Term Storage of Lipid-Like Nanoparticles for mRNA Delivery

The paper titled “Long-term Storage of Lipid-Like Nanoparticles for mRNA Delivery”, led by Pengxuan Zhao and published in Bioactive Materials, tackles the critical issue of nanoparticle stability during long-term storage. While LNPs have shown great promise for mRNA delivery, their clinical application is limited by the lack of a reliable storage method. The study’s innovation lies in developing storage conditions that preserve the stability of lipid-like nanoparticles (LLNs) and their ability to deliver mRNA effectively over time. The research demonstrates that adding cryoprotectants like sucrose and trehalose to LLNs enhances their stability during freezing and lyophilization, allowing them to maintain delivery efficiency even after storage in liquid nitrogen for three months. The study used in vitro assays and in vivo mouse models to measure mRNA delivery efficiency and found that LLNs stored under optimal conditions maintained high potency. The findings are significant for advancing the clinical use of LNPs by providing a reliable method for their long-term storage without compromising their mRNA delivery capabilities. This advancement is key for ensuring that LNP-based mRNA therapies can be stored and transported effectively, paving the way for widespread clinical applications.
Paper 3: The Effects of PEGylation on LNP-Based mRNA Delivery to the Eye

Led by Renee C. Ryals, “The Effects of PEGylation on LNP-Based mRNA Delivery to the Eye” was published in PLOS ONE. The study focuses on optimizing LNPs for gene therapy applications in the retina, particularly for retinal degenerative diseases. Traditional gene delivery methods often struggle to target retinal cells efficiently, but LNPs provide a promising alternative. The key innovation of this study is the modification of LNP formulations by adjusting the PEGylation levels. The study found that decreasing the PEG content in LNPs enhanced gene expression in retinal cells. This finding could improve the effectiveness of LNP-mediated mRNA delivery, especially for diseases like retinal degeneration. The study employed both subretinal and intravitreal injection techniques to assess the delivery and expression of luciferase mRNA, as well as the use of cre mRNA for gene editing. The findings showed that LNPs with lower PEG content (0.5%) resulted in better gene expression in retinal cells and provided valuable insights into the design of LNPs for ocular gene therapy. These results are significant because they suggest that fine-tuning LNP formulations can enhance their ability to deliver mRNA to the eye, expanding their potential for treating retinal diseases that currently have no effective treatments.
Paper 4: Ionizable Lipid Nanoparticles for In Utero mRNA Delivery

The paper “Ionizable Lipid Nanoparticles for In Utero mRNA Delivery”, led by researchers from institutions including Oregon Health & Science University, explores the potential of LNPs for prenatal genetic therapies. Published in Science Advances, the research focuses on using LNPs to deliver mRNA to fetuses in utero, a promising approach for treating genetic disorders diagnosed early in pregnancy. The innovation in this study lies in the development of LNPs that are capable of efficiently delivering mRNA to fetal tissues, including the liver, lungs, and intestines. The study found that certain LNP formulations could deliver therapeutic mRNA to fetal liver cells, where it induced the production of therapeutic proteins. This opens up the possibility of using LNPs for prenatal gene therapy, which could be used to treat genetic disorders before irreversible damage occurs. The study used mouse models to evaluate the distribution and efficiency of mRNA delivery to various fetal organs and demonstrated that LNPs can successfully cross the placental barrier. The significance of this research is profound, as it represents a major step forward in the field of gene therapy, offering the potential to treat genetic diseases before birth and potentially prevent lifelong health issues for affected infants.
Conclusion
In conclusion, lipid nanoparticles (LNPs) represent a transformative technology for mRNA delivery, with significant advancements in their design and application across various therapeutic fields. The research highlighted in these studies demonstrates the versatility and potential of LNPs, from enhancing CAR T cell therapies with safer and more efficient mRNA delivery to T cells, to overcoming the challenges of long-term storage for broader clinical use. Innovations like PEGylation adjustments and ionizable lipid formulations have enabled targeted and efficient delivery to specific tissues, including the retina and fetal organs, opening doors for new treatments in gene therapy. As these studies continue to evolve, LNPs are set to play a pivotal role in mRNA-based therapeutics, offering safer, more effective alternatives to traditional delivery systems while expanding the possibilities for treating genetic disorders, cancers, and diseases with previously limited therapeutic options. The continued optimization and understanding of LNP formulations will be crucial for advancing clinical applications and ensuring that mRNA therapies reach their full potential, benefiting patients worldwide.
