Editor: Sarah
A recent study, published in Molecular Cancer, presents a new approach to improving the effectiveness of hyperthermic intraperitoneal chemotherapy (HIPEC) for treating peritoneal metastases. The research focuses on the development of a metal-enriched HSP90 nanoinhibitor aimed at overcoming the heat resistance in tumor cells, a significant factor hindering the success of HIPEC.
Enhancing Tumor Cell Sensitivity to Heat and Chemotherapy
HIPEC, which combines heated chemotherapy and surgery, has been effective in treating peritoneal metastases, especially in colorectal cancer patients. However, heat shock proteins (HSPs), particularly HSP90, play a critical role in protecting tumor cells from the damaging effects of heat. The study introduces a bifunctional nanoinhibitor combining epigallocatechin gallate (EGCG) with manganese ions, specifically targeting HSP90 to inhibit its function and increase tumor cell susceptibility to heat-induced death and chemotherapy.
The researchers found that the nanoinhibitor not only impaired the heat resistance mechanisms in tumor cells but also induced pyroptosis, a form of inflammatory cell death. This led to the activation of immune responses, aiding in the elimination of metastatic tumor cells. Animal model experiments demonstrated that combining this nanoinhibitor with HIPEC resulted in a significantly higher tumor cell death rate and improved survival.
Contributions and Key Findings
- Development of the Nanoinhibitor: The research team designed a metal-enriched HSP90 nanoinhibitor, formed by combining EGCG and manganese ions using a flash nanocomplexation technique. This nanoinhibitor effectively inhibits HSP90 in tumor cells and enhances their susceptibility to heat stress, thereby increasing their vulnerability to chemotherapy.
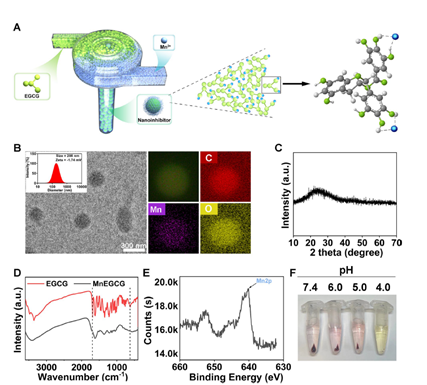
Figure 1: Synthesis and characterization of HSP90 nanoinhibitor
- Synergistic Effect with Heat: The nanoinhibitor, when combined with heat, induced oxidative stress and activated pyroptosis in tumor cells. This synergistic effect not only attacked the tumor directly but also mobilized the body’s immune system, enhancing the overall therapeutic outcome.
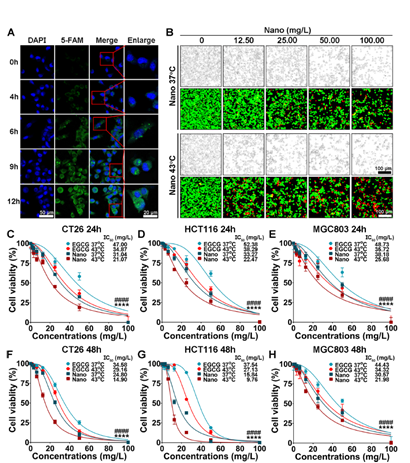
Figure 2: Cellular uptake and antiproliferation effect of the nanoinhibitor
- Activation of Immune Response: The study demonstrated that the nanoinhibitor induced immunogenic cell death (ICD) through pyroptosis. This process activated dendritic cells, leading to enhanced immune responses against the tumor. Tumor cells treated with the nanoinhibitor showed increased levels of calreticulin, HMGB1, and ATP, which are crucial for immune activation.
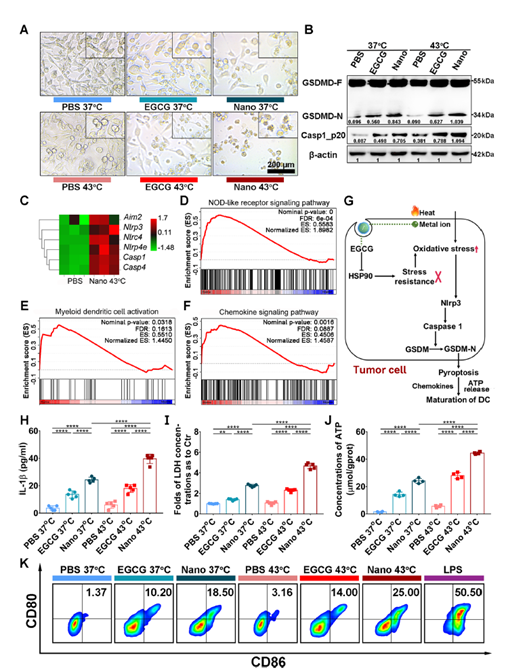
Figure 3: Tumor cell death and immunostimulation induced by nanoinhibitor in vitro.
- Improvement in Survival: In animal models, the combination of HIPEC with the nanoinhibitor significantly improved tumor suppression and increased survival rates. Notably, the nanoinhibitor-based HIPEC therapy exhibited a tumor inhibition rate of 74.6%, demonstrating its superior efficacy compared to traditional treatments.
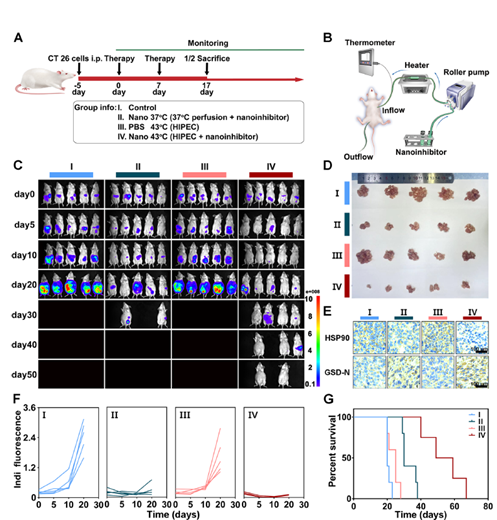
Figure 4: Antitumor effect of nanoinhibitor in vivo.
- Clinical Relevance: The findings suggest that this novel approach could offer a new strategy for treating patients with colorectal peritoneal metastases, particularly those who are unresponsive to conventional treatments. The next step is to initiate clinical trials to further explore the safety and efficacy of the nanoinhibitor in human patients.
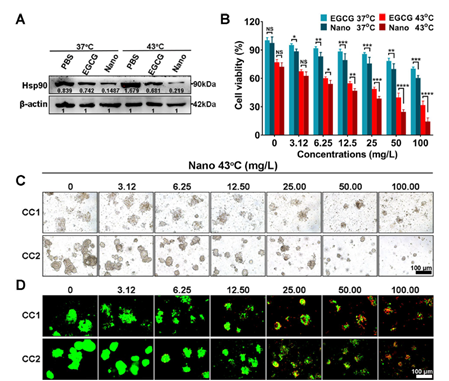
Figure 5: Translational research of nanoinhibitors in organoids.
Robust Research Methodology
The study utilized advanced techniques such as molecular docking, RNA sequencing, and in vivo experiments with mouse models to ensure the reliability and robustness of the results. Molecular docking revealed that EGCG binds to the C-terminal domain of HSP90, inhibiting its function. RNA sequencing and gene expression analysis demonstrated that the nanoinhibitor induced significant changes in genes associated with stress responses and immune activation.
Clinical Implications and Future Directions
The study suggests that the metal-enriched HSP90 nanoinhibitor could significantly enhance the efficacy of HIPEC, offering a potential therapeutic solution for peritoneal metastases in colorectal cancer patients. This approach may not only improve survival rates but also reduce the need for additional chemotherapy agents, thereby minimizing toxic side effects.
Further research is required to confirm these findings in clinical trials. If successful, this strategy could be extended to other types of peritoneal metastases and potentially lead to a broader application of nanotechnology in cancer treatment.
The research highlights the potential of combining nanotechnology and HIPEC to overcome the limitations of conventional cancer therapies, offering hope for more effective treatments in patients with advanced-stage colorectal cancer and other peritoneal metastases.
Reference
Wang, Qiang, et al. “Metal-Enriched HSP90 Nanoinhibitor Overcomes Heat Resistance in Hyperthermic Intraperitoneal Chemotherapy Used for Peritoneal Metastases.” Molecular Cancer, vol. 22, no. 95, 2023, https://doi.org/10.1186/s12943-023-01790-2.
