Editor: Nina
This study demonstrates that amphotericin B nano-assemblies, synthesized using a green method from mango pulp, significantly reduce toxicity while enhancing therapeutic efficacy against visceral leishmaniasis, presenting a promising alternative to conventional treatments.
Key Preview
Research Question
The study investigates whether amphotericin B (AmB) nano-assemblies can mitigate the drug’s inherent toxicity while enhancing its therapeutic efficacy in treating visceral leishmaniasis.
Research Design and Strategy
The research employs a green synthesis method to develop amphotericin B nano-assemblies (AmB-NA) and subsequently examines their pharmacokinetics, toxicity, and anti-leishmanial activity in vitro and in vivo.
Method
The study utilizes in vitro assays to evaluate the toxicity of AmB-NA against red blood cells and macrophages, alongside in vivo experiments in BALB/c mice infected with Leishmania donovani.
Key Results
AmB-NA demonstrated significantly reduced toxicity (minimal hemolysis) compared to conventional formulations, while effectively lowering parasite burdens in mouse models, indicating a promising alternative to existing treatments.
Significance of the Research
This study could pave the way for a more effective and safer treatment for visceral leishmaniasis, a neglected tropical disease that poses severe health risks in endemic regions.
Introduction
Visceral leishmaniasis (VL) is a parasitic disease caused by Leishmania donovani, primarily affecting populations in South Asia, including India and Bangladesh. The disease remains one of the most neglected tropical diseases, often leading to severe morbidity and mortality. Current treatment options, particularly amphotericin B, are hindered by significant toxicity and the challenges of drug resistance. While lipid formulations like AmBisome have improved safety profiles, their high cost and limited availability restrict their use.
Recent research has focused on developing innovative drug formulations that can enhance the efficacy of existing treatments while minimizing toxic effects. The current study introduces a novel approach by using green synthesis techniques to create nano-assembled forms of amphotericin B (AmB-NA), hypothesizing that these formulations can improve pharmacological outcomes while reducing toxicity.
Research Team and Objective
The study was conducted by a multidisciplinary research team from Aligarh Muslim University and the Rajendra Memorial Research Institute of Medical Sciences, comprising experts in biotechnology, microbiology, and immunology. The primary objective was to synthesize AmB-NA via a plant-based approach using Mangifera indica (mango) pulp, and to evaluate its safety and efficacy in experimental models of visceral leishmaniasis.
Experimental Process
1. Synthesis of Amphotericin B Nano-Assembles (AmB-NA)
Key Steps:
- Amphotericin B (AmB) was mixed with a 30% w/v stock solution of Mangifera indica (mango) pulp extract in a ratio of 1:1.
- The mixture was stirred at room temperature for 24 hours to facilitate the formation of nano-assembled structures.
- The resulting suspension was centrifuged at 20,000 × g for 20 minutes to pellet the AmB-NA, followed by washing the pellet with phosphate-buffered saline (PBS) and dialysis for 24 hours to remove any unbound AmB.
Results and Key Data:
- The average diameter of the synthesized AmB-NA was determined to be approximately 97 nm, with a zeta potential of -34.2 mV, indicating a stable formulation.
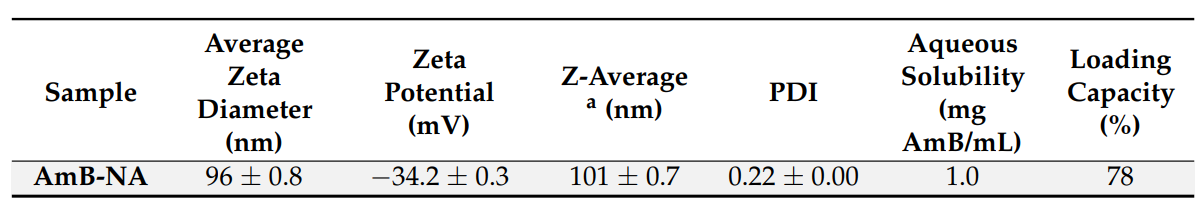
Table 1. Particle size and zeta potential of AmB-NA
- Spectroscopic analysis confirmed the characteristic UV absorption of the super-aggregated form of AmB.
Significance of the Result:
- The successful synthesis of AmB-NA through a green method suggests a viable alternative to conventional formulations that can reduce toxicity while maintaining therapeutic efficacy.
- The stability of the nano-assemblies is crucial for enhancing the bioavailability of AmB, allowing for controlled release and minimizing side effects.
Key Innovations:
- The use of a plant-based extract (mango pulp) for the synthesis of AmB nano-assemblies represents a novel approach in drug formulation, aligning with environmentally friendly practices.
- The resulting nano-assemblies exhibited improved physicochemical properties compared to traditional formulations.
2. Toxicity Evaluation
Key Steps:
- The hemolytic activity of AmB-NA was assessed by incubating isolated red blood cells (RBCs) from BALB/c mice with varying concentrations of AmB-NA for 24 hours.
- The extent of hemolysis was quantified by measuring the release of hemoglobin using spectrophotometry at 576 nm.
Results and Key Data:
- AmB-NA demonstrated less than 15% hemolysis at concentrations up to 100 µg/mL, significantly lower than conventional formulations such as Fungizone, which exhibited nearly complete hemolysis at similar concentrations.
Significance of the Result:
- The reduced hemolytic activity of AmB-NA highlights its potential as a safer alternative for treating conditions such as visceral leishmaniasis, addressing one of the primary concerns associated with traditional AmB formulations.
Key Innovations:
- The innovative use of nano-assembly techniques resulted in a formulation that minimizes toxicity while retaining therapeutic effectiveness, showcasing the potential for improved patient safety.
3. In Vitro Efficacy against Leishmania donovani
Key Steps:
- The leishmanicidal activity of AmB-NA was tested against both promastigotes and amastigotes of Leishmania donovani through MTT assays and Giemsa staining of infected macrophages.
- Concentrations of AmB-NA were applied, and the viability of the parasites was assessed after 48 hours.
Results and Key Data:
- AmB-NA showed IC50 values of 0.05 µM and 0.06 µM against promastigotes and amastigotes, respectively, indicating potent anti-leishmanial activity, significantly outperforming both free AmB and AmBisome.
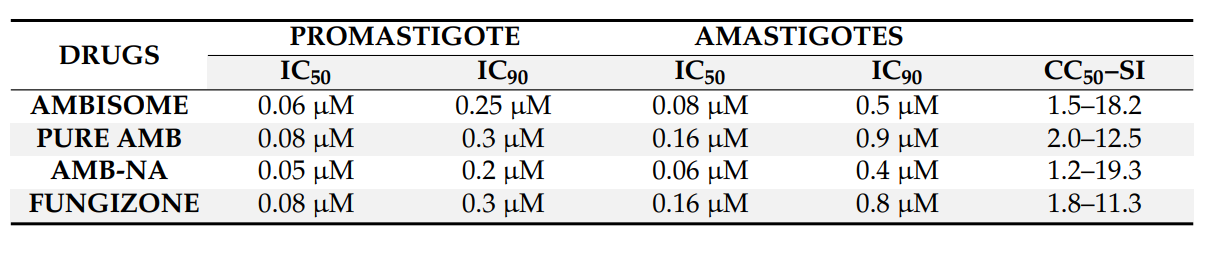
Table 2. In vitro antileishmanial activities (IC50 and IC90) of AmB-NA compared to those of Fungizone and AmBisome in macrophages infected with L. donovani amastigotes, observed after 48 h of incubation. CC50 was estimated after 48 h of drug treatment to macrophages. The SI represent the selectivity index calculated by dividing the CC50 by the IC50 value obtained after treatment of cultured macrophages after 48 h.
Significance of the Result:
- The superior efficacy of AmB-NA against both life stages of Leishmania suggests its potential as a more effective treatment option, which is particularly important in the context of rising drug resistance.
Key Innovations:
- The formulation’s ability to target both promastigotes and amastigotes enhances its therapeutic applicability, addressing the multifaceted nature of leishmaniasis.
4. In Vivo Efficacy in BALB/c Mouse Model
Key Steps:
- BALB/c mice were infected with L. donovani and subsequently treated with AmB-NA, AmBisome, and free AmB at specified doses.
- Post-treatment, the parasitic burden was evaluated in the spleen, liver, and bone marrow via Giemsa staining.
Results and Key Data:
- Mice treated with AmB-NA showed a significant reduction in parasite load, with counts of 16 ± 5 and 5 ± 2 amastigotes per 1000 spleen cells for single and double doses, respectively, compared to higher counts in untreated controls.
Significance of the Result:
- The substantial decrease in parasitic burden in treated mice indicates the potential of AmB-NA to effectively clear visceral leishmaniasis, presenting an important advancement in treatment strategies.
Key Innovations:
- The study’s innovative approach of using a dual-dose regimen with AmB-NA shows promise in enhancing treatment outcomes, potentially leading to more efficient therapeutic protocols for visceral leishmaniasis.
5. Immune Response Assessment
Key Steps:
- The delayed type hypersensitivity (DTH) response was evaluated in treated mice by measuring footpad swelling after administration of soluble leishmanial antigen (SLA).
- Serum samples were collected to measure IgG isotype levels and cytokine production via ELISA.
Results and Key Data:
- A robust DTH response was observed in AmB-NA-treated groups, with significant footpad swelling compared to controls. The IgG2a/IgG1 ratio was also significantly higher in these groups, indicating a Th1-biased immune response.
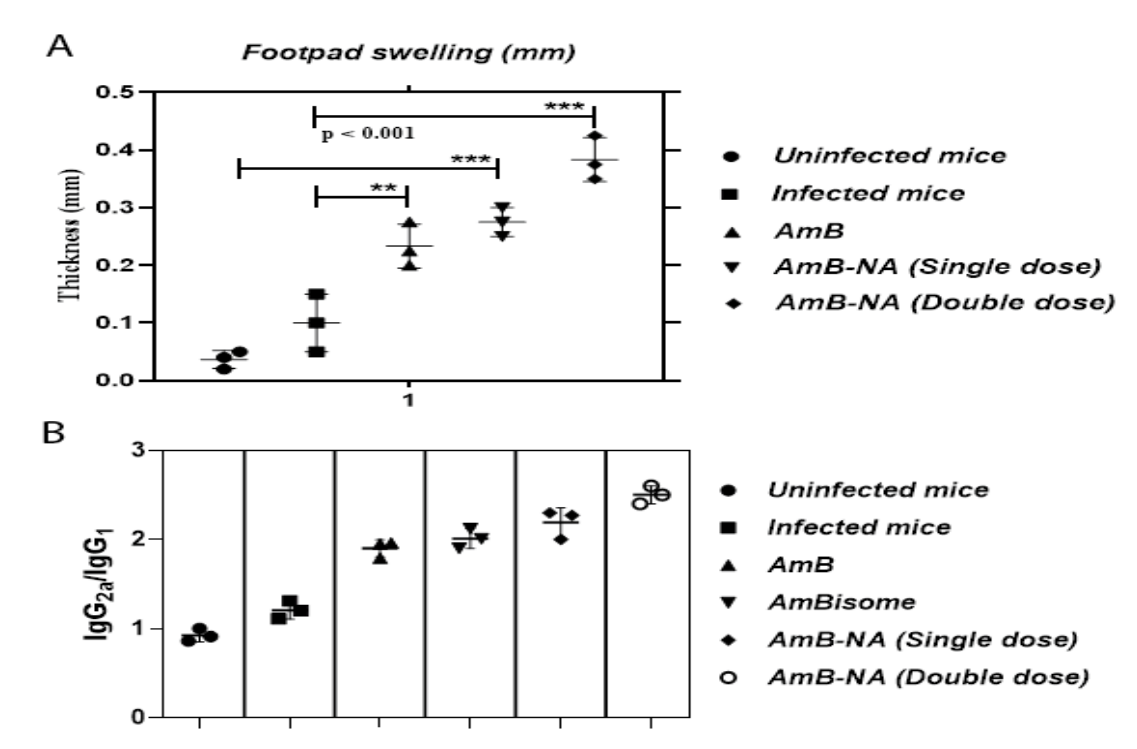
Figure 1. Immunomodulatory potential of the AmB-NA in the L. donovani-infected BALB/c mice. The L. donovani-infected animals were treated with various as-synthesized AmB-NA formulations following a published protocol described in the method. The splenocytes of the cured animals were isolated after 4-week post-treatment as described in the method.
Significance of the Result:
- The strong immune response elicited by AmB-NA treatment suggests not only effective parasite clearance but also the potential for long-lasting immunity against future infections.
Key Innovations:
- This study illustrates the ability of AmB-NA to modulate immune responses favorably, enhancing protective immunity while effectively combating the disease.
Conclusion
The research presents compelling evidence that amphotericin B nano-assemblies, synthesized through a green method, offer a dual advantage of reduced toxicity and enhanced therapeutic efficacy against visceral leishmaniasis. The findings suggest that these formulations could serve as a viable alternative to existing AmB treatments, potentially improving patient outcomes in endemic regions. However, the study acknowledges limitations in terms of long-term efficacy and safety, prompting recommendations for further research to explore the clinical applicability of AmB-NA in diverse populations.
The study ultimately contributes to the ongoing search for innovative solutions to combat neglected diseases, emphasizing the potential of nanotechnology in drug development.
Reference
Jamal, Fauzia, et al. “Amphotericin B nano-assemblies circumvent intrinsic toxicity and ensure superior protection in experimental visceral leishmaniasis with feeble toxic manifestation.” Vaccines 11.1 (2023): 100.
