Editor: Nina
This study presents a novel class of charge-altering releasable transporters (bAC CARTs) that significantly enhance the delivery of mRNA to primary T lymphocytes in vitro and exhibit selective organ tropism in vivo, offering promising advancements for targeted gene therapies and immunotherapies.
Key Preview
Research Question
This study addresses the challenge of enhancing mRNA delivery systems, especially for difficult-to-transfect cells such as primary T lymphocytes. The research aims to improve the efficacy and selectivity of mRNA delivery technologies that are critical for therapeutic applications, including vaccines and gene therapies.
Research Design and Strategy
The researchers developed a new class of charge-altering releasable transporters (CARTs) with a distinct beta-amido carbonate backbone (bAC) to improve mRNA transfection efficiency and organ tropism in vivo. The study utilized a combination of in vitro and in vivo experiments to evaluate the performance of these bAC-CARTs against existing delivery systems.
Method
The research involved synthesizing a library of bAC CARTs, assessing their transfection efficiency in Jurkat cells and primary T lymphocytes, and evaluating their in vivo tropism in murine models. Various experimental techniques, including flow cytometry, bioluminescence imaging, and confocal microscopy, were employed to analyze the effectiveness of these transporters.
Key Results
The bAC CARTs demonstrated up to 70% transfection efficiency in primary T cells and significant spleen tropism (up to 97%) in vivo, outperforming previous delivery systems. The study also found that bAC CARTs could deliver mRNA with minimal toxicity and maintained the functionality of T cells post-transfection.
Significance of the Research
This research represents a significant advancement in mRNA delivery systems, providing a promising approach for targeted gene therapies and immunotherapies. The development of bAC CARTs could potentially improve the effectiveness of mRNA-based treatments while minimizing off-target effects.
Introduction
The field of mRNA therapeutics has rapidly evolved, particularly highlighted by the success of COVID-19 vaccines. However, the delivery of mRNA, especially to hard-to-transfect cells like primary T lymphocytes, remains a significant challenge. Current strategies, including lipid nanoparticles (LNPs) and viral vectors, often fall short in efficacy and selectivity. This study introduces a new type of charge-altering releasable transporters (CARTs) that utilize a beta-amido carbonate backbone to enhance mRNA delivery. The study aims to tackle the limitations of existing delivery systems, particularly their inability to efficiently target and transfect T lymphocytes, which play a critical role in immune responses and cancer immunotherapy.
Research Team and Objective
The research team consists of prominent scientists from Stanford University, including Zhijian Li, Laura Amaya, Ruoxi Pi, Sean K. Wang, Alok Ranjan, Robert M. Waymouth, Catherine A. Blish, Howard Y. Chang, and Paul A. Wender. Conducted between January 2023 and October 2023, their study titled “Charge-altering releasable transporters enhance mRNA delivery in vitro and exhibit in vivo tropism” was published in Nature Communications. The objective was to develop effective delivery systems for mRNA that could enhance transfection rates, particularly in T cells, and exhibit selective organ tropism.
Experimental Process
1. Synthesis of bAC CARTs
Key Steps:
- A library of 24 bAC CARTs was synthesized through a step-economical organocatalytic ring-opening polymerization (OROP) method.
- Each CART was designed with varying lipid components and backbone lengths to explore their impact on transfection efficiency.
Result and Key Data:
- The successful synthesis of bAC CARTs was confirmed via 1H-NMR and gel permeation chromatography (GPC), yielding CARTs with polydispersity indices ranging from 1.14 to 1.37.
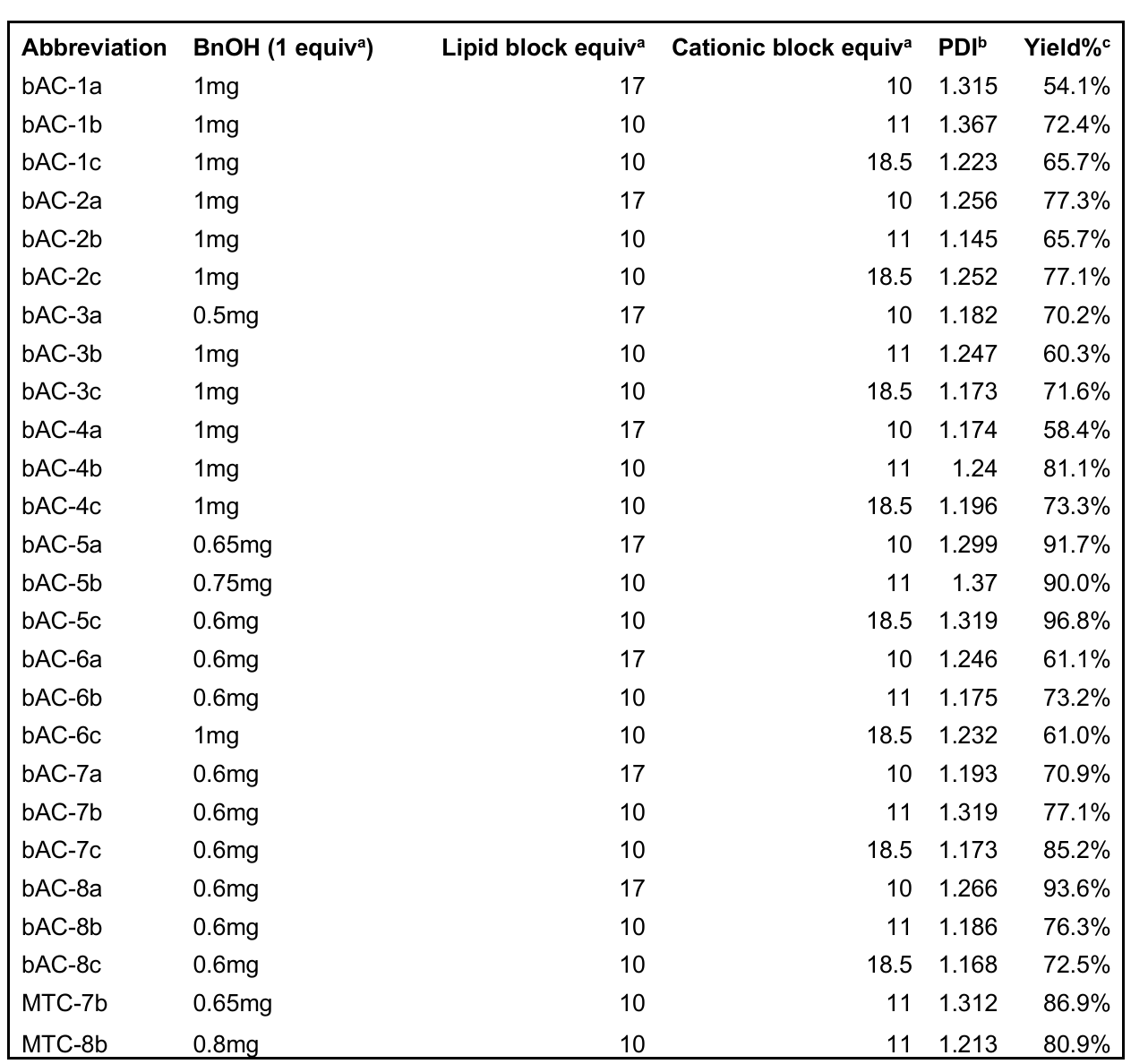
Table 1. Polymerization conditions of CARTs.aMole equivalent, bPolydispersity index (PDI) was obtained from gel permeation chromatography (GPC), cYields of polymers were calculated from mass recovered after dialysis.
Significance of the Result:
- This synthesis allowed for a systematic exploration of structural variations to improve mRNA delivery, setting the stage for subsequent evaluations of their transfection capabilities.
Key Innovations:
- The introduction of a novel bAC backbone, which differs in composition and side chain spacing from previously used polymeric backbones, aimed to enhance the stability and functionality of the mRNA delivery system.
2. In Vitro Transfection Tests in Jurkat Cells
Key Steps:
- The synthesized bAC CARTs were mixed with eGFP mRNA at an acidic pH (5.5) to form complexes, which were subsequently introduced to Jurkat cells.
- Flow cytometry was used to assess transfection efficiency 24 hours post-transfection, measuring the percentage of eGFP-positive cells.
Result and Key Data:
- The bAC-1a CART achieved over 70% transfection efficiency in Jurkat cells, outperforming the previously established MTC-1a (8%) and ONA CART (46%) systems.
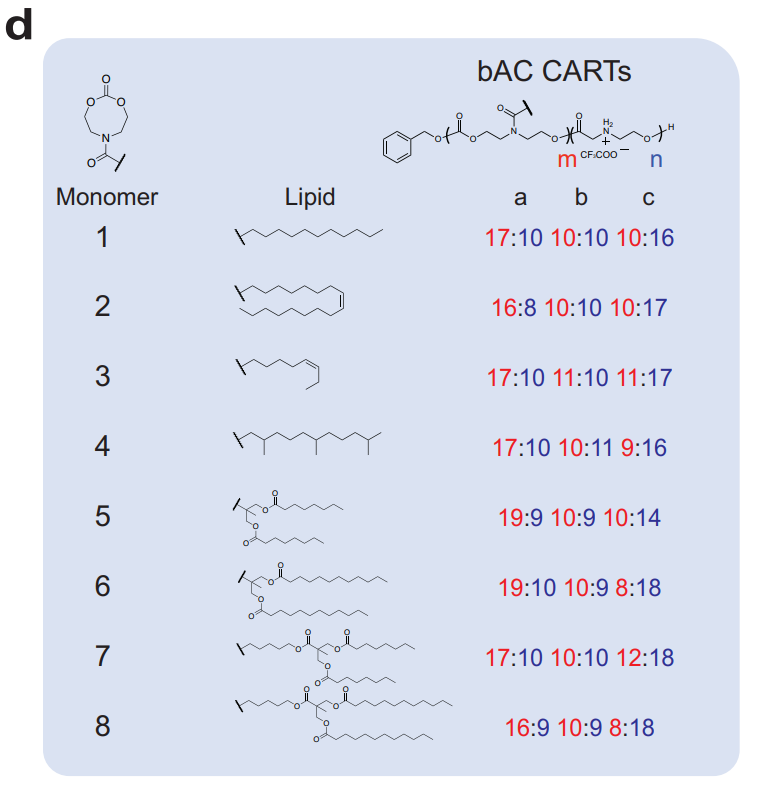
Figure 1. Chemical structures of bAC CART library.
Significance of the Result:
- Demonstrating high transfection rates in a T cell line underscores the potential of bAC CARTs for effective mRNA delivery, crucial for therapeutic applications in immunology.
Key Innovations:
- The bAC CARTs exhibited a significantly enhanced performance compared to existing systems, marking a pivotal advancement in the development of mRNA delivery technologies.
3. Evaluation of Primary T Lymphocyte Transfection
Key Steps:
- Freshly isolated primary T cells were transfected using the optimized bAC CARTs, following a similar protocol as the Jurkat cell tests.
- The transfection efficiency was again quantified using flow cytometry to determine the percentage of eGFP-positive T cells.
Result and Key Data:
- The bAC-7b CART resulted in approximately 65% transfection efficiency in primary T lymphocytes, which is a marked improvement compared to the ONA CART, which only achieved around 10% transfection.
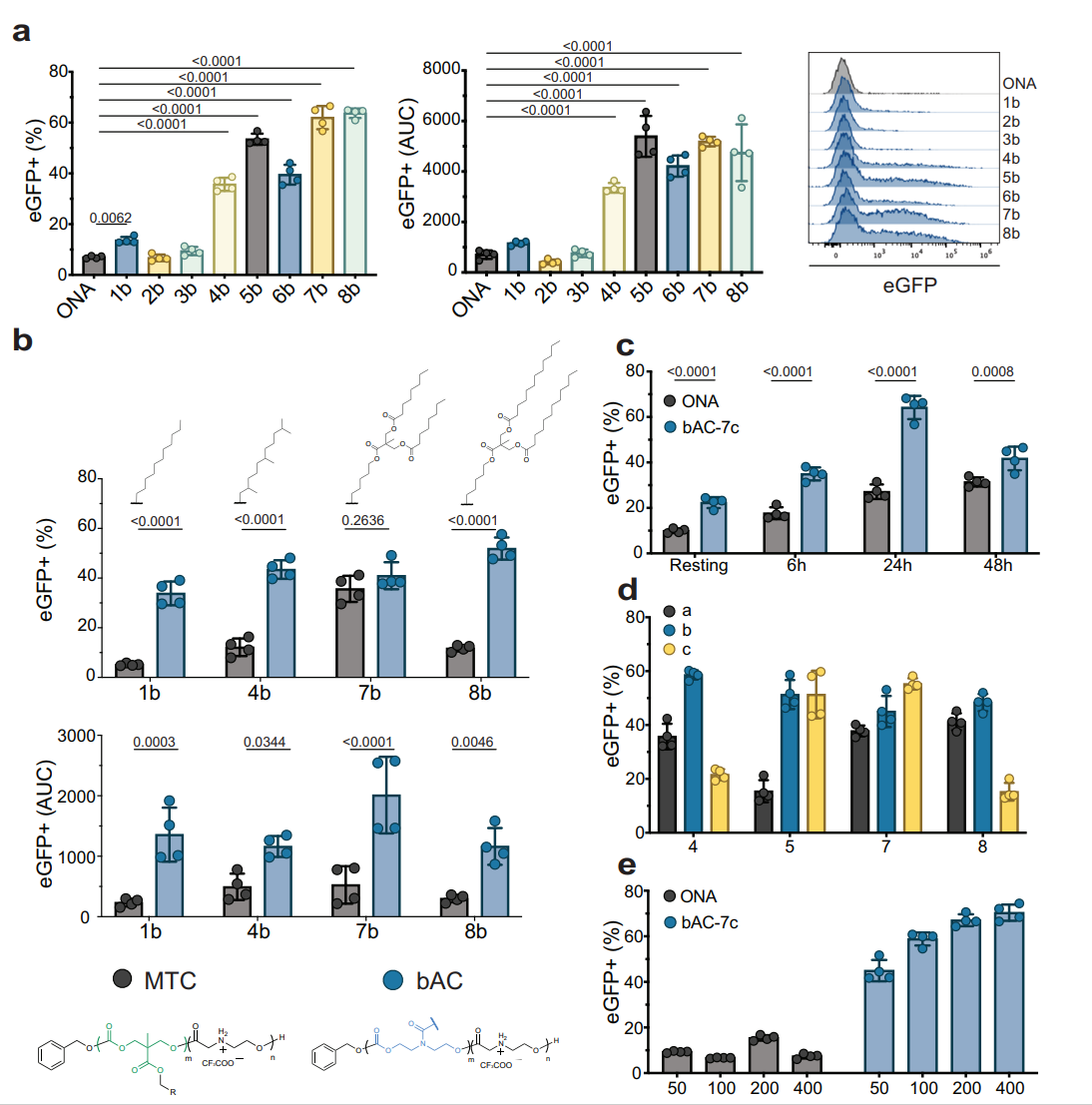
Figure 2. bAC polymeric backbone improves primary T lymphocyte transfection
Significance of the Result:
- This outcome is significant as it indicates that bAC CARTs are effective in transfecting primary T lymphocytes, a cell type critical for immune responses and therapeutic applications.
Key Innovations:
- The ability of bAC CARTs to transfect primary T cells efficiently without the need for targeting ligands represents a breakthrough in mRNA delivery systems, enhancing their applicability in vivo.
4. In Vivo Delivery and Tropism Assessment
Key Steps:
- Mice were injected intravenously with bAC CARTs complexed with luciferase mRNA to assess in vivo transfection efficiency and organ tropism.
- Bioluminescence imaging was performed 6 hours post-injection to evaluate luciferase expression in various organs.
Result and Key Data:
- The bAC-4b and bAC-7c CARTs demonstrated up to 97% tropism for the spleen, with bAC-4b showing 2.5-fold higher luciferase expression than the best-performing MTC analog.
Significance of the Result:
- This result highlights the organ-specific delivery capabilities of bAC CARTs, which is essential for targeting therapies to specific tissues while minimizing off-target effects.
Key Innovations:
- The ability to achieve such high spleen tropism without targeting ligands is a significant advancement, paving the way for more effective and targeted immunotherapies.
5. Functional Analysis of Transfected T Cells
Key Steps:
- Following transfection, the functionality of the primary T cells was assessed through activation marker analysis (CD25 and CD69) and cytokine production (IFN-γ and TNF-α) upon stimulation.
Result and Key Data:
- Transfected T cells exhibited activation levels and cytokine production comparable to untransfected controls, indicating that the transfection process did not impair T cell functionality.
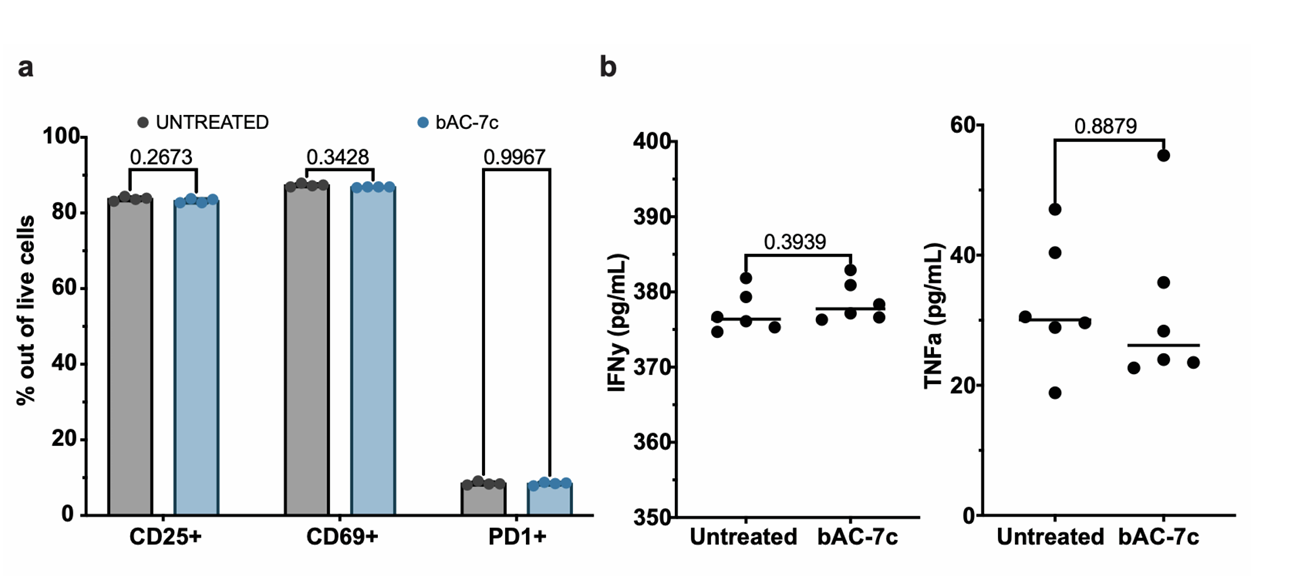
Figure 3.Activation and functionality of primary human T cells after bAC-7c CART transfection. a) Protein expression levels of activation and exhaustion markers by flow cytometry (n=4, bars represent mean with SD). Statistical significance was calculated using 2way ANOVA. b) Inflammatory cytokine levels measured by ELISA (n=6, bar represents median).
Significance of the Result:
- These findings are crucial for clinical applications, as they confirm that the transfection does not compromise the immune capabilities of T cells, essential for effective therapeutic outcomes.
Key Innovations:
- Demonstrating that T cells remain functional post-transfection with bAC CARTs enhances the potential for using these systems in therapies such as CAR-T cell engineering.
6. Toxicology and Safety Assessment
Key Steps:
- Comprehensive toxicology assessments were performed post-administration of bAC CART/mRNA formulations in mice, analyzing blood samples for liver and kidney function indicators.
Result and Key Data:
- Blood metabolite levels were comparable to those of FDA-approved MC3 LNP formulations, showing no signs of acute toxicity or alterations in blood counts.
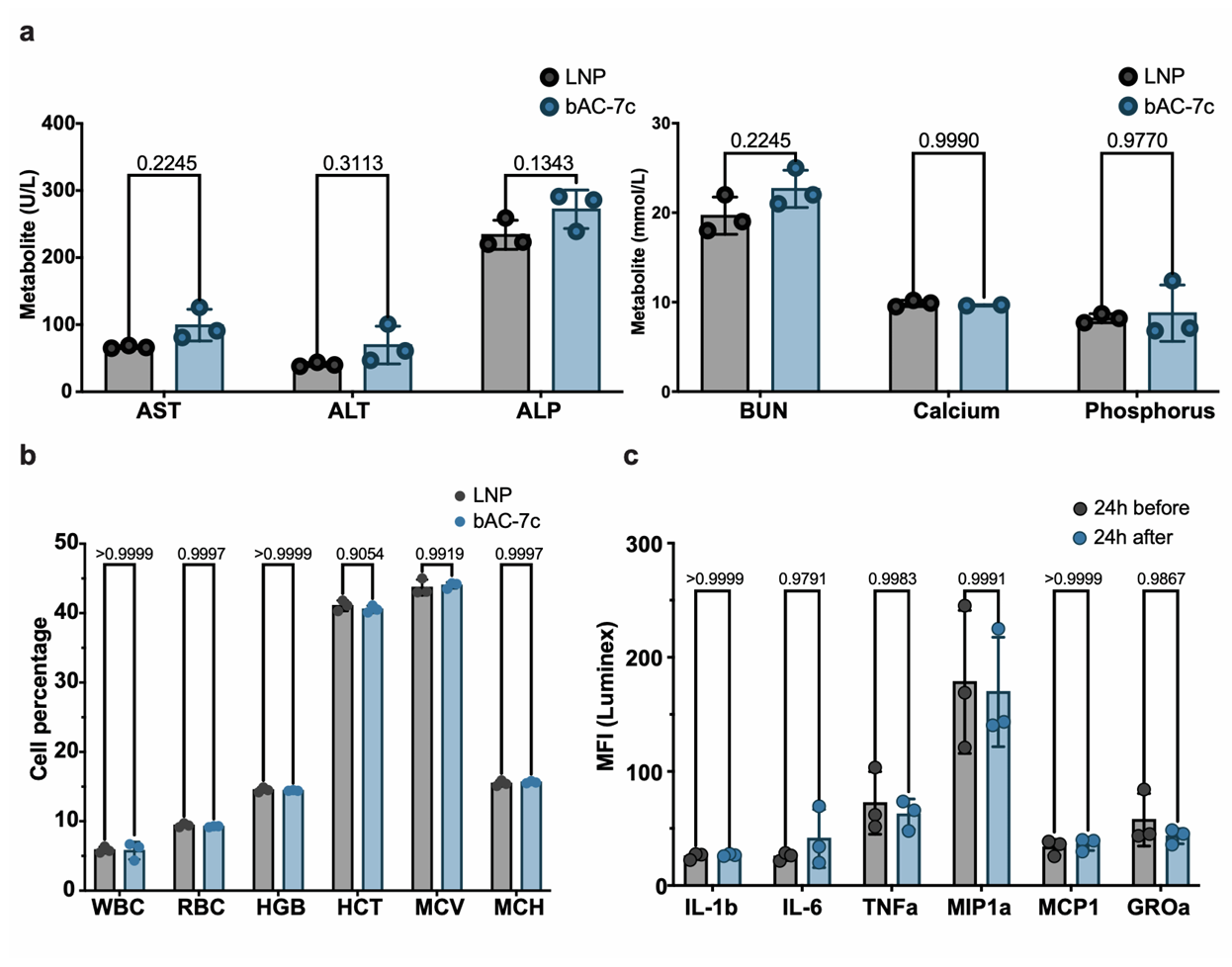
Figure 4. Preclinical toxicology blood analysis 24 hours after delivery of 7.5 mg of luciferase mRNA formulated with bAC CART compared to MC3 LNP. a) Blood metabolites, b) blood counts and c) inflammatory cytokines 24 hours before and after delivery of luciferase mRNA with bAC-7c CART (n=3, bars represent mean with SD). Statistical significance was calculated using 2way ANOVA.
Significance of the Result:
- This safety profile is critical for advancing bAC CARTs toward clinical applications, as it suggests that the system is well-tolerated and does not induce adverse effects.
Key Innovations:
- The assessment of safety and tolerability alongside efficacy demonstrates a comprehensive approach to developing new mRNA delivery systems, which is vital for regulatory approval and clinical use.
Conclusion
The study successfully demonstrated the potential of bAC CARTs as a new class of mRNA delivery systems, achieving high transfection rates in primary T lymphocytes and selective spleen tropism in vivo. These findings imply that bAC CARTs could significantly enhance the development of mRNA-based therapies, particularly in the fields of cancer immunotherapy and vaccine development. However, limitations such as the need for further optimization in terms of payload capacity and long-term stability were acknowledged. Future research directions may include exploring the use of bAC CARTs for delivering other polyanionic molecules, such as siRNA and plasmid DNA, to broaden their therapeutic applications.
Reference
Li, Zhijian, et al. “Charge-altering releasable transporters enhance mRNA delivery in vitro and exhibit in vivo tropism.” Nature Communications, vol. 14, no. 6983, 2023, doi:10.1038/s41467-023-42672-x.
