Author: Tiffany
Researchers have developed a novel nanocarrier system, ZnO@ZIF-8, that enhances the delivery and effectiveness of the antibiotic ciprofloxacin against antibiotic-resistant bacterial strains, potentially transforming treatment strategies for resistant infections.
Key Highlights
- Research Question:
Can a new nanocarrier system improve the delivery and efficacy of ciprofloxacin against resistant bacteria? - Research Difficulties:
Achieving efficient drug delivery while overcoming the challenges of antimicrobial resistance and ensuring minimal side effects. - Key Findings:
The ZnO@ZIF-8 nanocarrier achieved a ciprofloxacin loading efficiency of 46% and demonstrated effective antibacterial activity at significantly lower concentrations than conventional treatments. - Innovative Aspects:
The study introduces a novel synthesis method for ZnO@ZIF-8, which combines the antimicrobial properties of zinc oxide with the drug-loading capabilities of zeolitic imidazolate frameworks (ZIFs). - Importance of the Study:
This research addresses the urgent need for new strategies to combat antimicrobial resistance, offering a promising approach for prolonged and effective antibiotic treatment.
Impact of Antimicrobial Resistance and Nanocarrier Development
Antimicrobial resistance (AMR) is a growing global threat, with resistant bacteria causing over 700,000 deaths annually and projected to rise to 10 million by 2050. This crisis is exacerbated by the misuse of antibiotics, leading to the emergence of multidrug-resistant strains. Symptoms of infections caused by resistant strains include prolonged illness, increased mortality, and complications that can significantly impact public health and healthcare costs. Current therapeutic options often fail due to poor drug delivery, leading to inadequate therapeutic effects and increased side effects. Traditional antibiotics may not penetrate biofilms or reach effective concentrations at infection sites, underscoring the need for innovative delivery systems.
Objectives of ZnO@ZIF-8 Nanocarrier Research for Ciprofloxacin Delivery
Conducted by a team from the São Paulo State University (UNESP), including researchers Bruno Altran Costa, Marina Paiva Abuçafy, and others, the study was published in January 2023 in the journal Pharmaceutics.
The primary aim of this study is to investigate the potential of a novel nanocarrier system, ZnO@ZIF-8, in improving the loading, release, and antimicrobial efficacy of ciprofloxacin against resistant bacterial strains. The specific objectives of the research include:
- Synthesize and characterize the ZnO@ZIF-8 nanocarrier, assessing its structural integrity, size, morphology, and surface properties.
- Evaluate the efficiency of ciprofloxacin loading into the ZnO@ZIF-8 nanocarrier to achieve high entrapment efficiency.
- Assess the release kinetics of ciprofloxacin from the ZnO@ZIF-8 nanocarrier in simulated physiological conditions.
- Evaluate the antibacterial activity of the ZnO@ZIF-8 nanocarrier and ciprofloxacin against resistant strains of Staphylococcus aureus and Pseudomonas aeruginosa.
- Explore the potential of the ZnO@ZIF-8 system as an alternative strategy in combating antimicrobial resistance.
Experimental Approach and Results of Ciprofloxacin Loading and Antibacterial Activity
Experimental Process Outline
- Synthesis of ZnO nanoparticles.
- Surface modification of ZnO nanoparticles with (3-Glycidyloxypropyl) trimethoxysilane (GPTMS).
- Synthesis of ZIF-8 nanoparticles using zinc nitrate and 2-methylimidazole.
- Synthesis of ZnO@ZIF-8 nanoparticles by combining ZnO and ZIF-8.
- Characterization of synthesized nanoparticles using XRD, SEM, FTIR, and TGA.
- Evaluation of ciprofloxacin loading into ZnO@ZIF-8.
- Assessment of ciprofloxacin release profile from ZnO@ZIF-8.
- Evaluation of antibacterial activity through minimum inhibitory concentration (MIC) tests against Staphylococcus aureus and Pseudomonas aeruginosa.
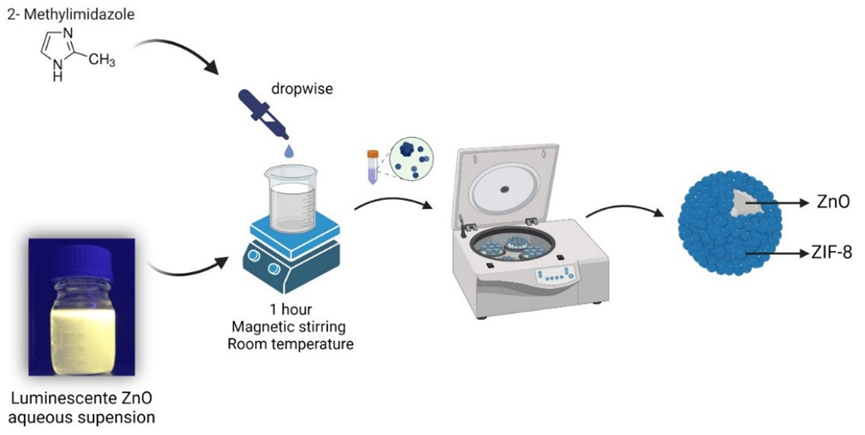
Figure 1. Schematic of ZnO@ZIF-8 NPs formation.
Key Experiments Introduction and Results
- Ciprofloxacin Loading Efficiency:
Procedure: To evaluate the potential application of ZnO@ZIF-8 as an antimicrobial system, the conventional antimicrobial drug ciprofloxacin was loaded onto ZnO@ZIF-8 by an impregnation method. A total of 15 mg of ZnO@ZIF-8 was soaked in 30 mL of an ethanolic solution of ciprofloxacin (0.5 mg mL−1) under stirring at room temperature. Aliquots were collected at different times (12 h, 24 h, and 48 h) to evaluate the best entrapment efficiency. The drug in the supernatant was measured by UV–vis spectroscopy at 273 nm, and the drug entrapment efficiency (EE%) was calculated using the following equation: Entrapment efficiency (EE%) = (weight of CIP in ZnO@ZIF-8 / weight of CIP fed initially) × 100
Results: After 12 hours, there was an average concentration of drug incorporation of 46%, which is a good percentage of incorporation when related to other studies in the literature, mainly of poorly soluble drugs. There was no statistically significant difference between the incorporation times of 12, 24, and 48 h, indicating that 12 h of incorporation in ethanol is sufficient to reach the plateau of loading. - Ciprofloxacin Release Profile:
Procedure: The release profile of ciprofloxacin from ZnO@ZIF-8 was acquired using simulated physiological medium (PBS buffered solution) at pH 7.4, representing physiological conditions. CIP-ZnO@ZIF-8 was suspended in PBS solution (30 mL) and incubated at 37 °C under constant stirring. Aliquots were removed at various time points (0.5, 1, 2, 3, 4, 6, 8, 12, 24, 36, 48, 72, and 96 h) and quantified indirectly by UV–vis spectroscopy at 273 nm.
Results: A tiny burst release at the early stage of the delivery was observed, with around 10% of the CIP released from the ZnO@ZIF-8 within the first hour. The trial showed a high rate of release in the first 12 h, with 37% of the drug released. After 4 days of experimentation, 67% of the drug was released, suggesting a prolonged release profile that may be beneficial for antimicrobial treatment.
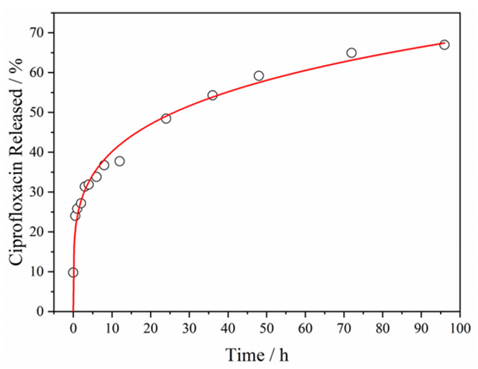
Figure 2. Profile of CIP release from ZnO@ZIF-8 into PBS solution at pH 7.4, at 37 ◦C. The data were fit to the Korsmeyer–Peppas equation, as indicated by the red line.
- Antibacterial Activity Assessment:
Procedure: Minimum inhibitory concentration (MIC) was performed to assess the antibacterial activity of ZIF-8, ZnO, ZnO@ZIF-8, and CIP-ZnO@ZIF-8 against Staphylococcus aureus and Pseudomonas aeruginosa. The bacterial strains were standardized according to the Clinical and Laboratory Standards Institute (CLSI) protocol. A microdilution of base 2 in Müeller–Hinton broth of the studied samples was performed at initial concentrations of 1 mg mL−1 and 5 mg mL−1, respectively.
Results: The MIC for ZIF-8 was found to be >1 mg mL−1 against S. aureus and >5 mg mL−1 against P. aeruginosa. The MIC for ZnO was 0.0652 mg mL−1 for S. aureus and 0.5 mg mL−1 for P. aeruginosa. The ZnO@ZIF-8 showed a MIC of 0.0625 mg mL−1 against S. aureus and 2.5 mg mL−1 against P. aeruginosa. Notably, the MIC for ciprofloxacin-loaded ZnO@ZIF-8 was significantly lower at 0.0062 mg mL−1 against S. aureus and 0.0125 mg mL−1 against P. aeruginosa, reflecting a 10-fold and 200-fold increase in antimicrobial activity, respectively.
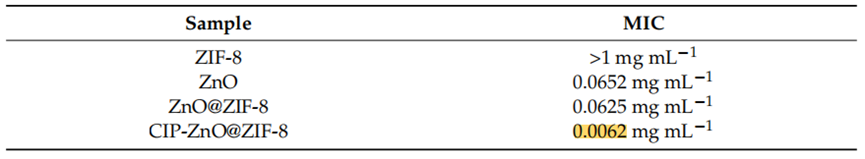
Table 1. MIC of samples against S. aureus (Gram-positive bacteria) (n = 3).
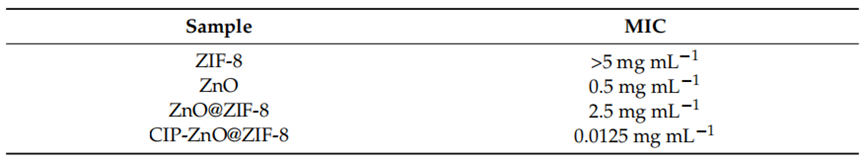
Table 2. MIC of samples against P. aeruginosa (Gram-negative bacteria) (n = 3).
These results indicate that the incorporation of ciprofloxacin into the ZnO@ZIF-8 system substantially enhances its antibacterial efficacy, making it a promising candidate for combating resistant bacterial strains.
Significance of Findings and Potential Applications in Overcoming Resistance
This study successfully developed a novel ZnO@ZIF-8 nanocarrier system that significantly enhances the loading, release, and antimicrobial efficacy of ciprofloxacin against resistant bacterial strains. Key findings include the achievement of a ciprofloxacin loading efficiency of 46%, a controlled release profile with 67% of the drug released over 96 hours, and impressive antibacterial activity characterized by minimum inhibitory concentrations (MICs) of 0.0062 mg mL−1 against Staphylococcus aureus and 0.0125 mg mL−1 against Pseudomonas aeruginosa. These results demonstrate the potential of the ZnO@ZIF-8 system to considerably reduce the dosage of ciprofloxacin required for effective treatment, thereby addressing the critical issue of antimicrobial resistance. The innovation of this research lies in the unique combination of ZnO nanoparticles with ZIF-8, creating a multifunctional nanocarrier that leverages the strengths of both components. The study provides a promising approach to optimize antibiotic delivery and effectiveness, which is crucial in the fight against increasing rates of bacterial resistance.
The researchers emphasize that the successful synthesis of ZnO@ZIF-8 not only preserves the physicochemical properties of ZnO and ZIF-8 but also facilitates the incorporation of ciprofloxacin. The research highlights the potential of this nanocarrier system to provide a controlled release mechanism and potent antibacterial activity against both Gram-positive and Gram-negative bacteria. This innovative system may serve as an alternative strategy to mitigate microbial resistance, combining multiple mechanisms of action that can enhance the therapeutic efficacy of existing antibiotics. The findings underscore the importance of exploring nanomaterials as versatile platforms in the development of future antimicrobial therapies.
Reference:
Costa, Bruno Altran, et al. “ZnO@ ZIF-8 nanoparticles as nanocarrier of ciprofloxacin for antimicrobial activity.” Pharmaceutics 15.1 (2023): 259.
