Editor: Sarah
Ischemic stroke (IS) is a major cause of death and long-term disability around the world. It results from restricted blood flow to the brain, leading to the death of brain cells in specific areas. The prevalence of IS is increasing globally, and it poses a significant economic burden due to the prolonged recovery periods and complications associated with stroke. Traditional treatments for IS often face significant challenges, including limited drug accumulation in the ischemic brain regions and potential systemic side effects from the drugs administered. In response to these challenges, the study titled “Application of Stimuli-Responsive Nanomedicines for the Treatment of Ischemic Stroke” introduces a novel approach that uses nanomedicines capable of responding to specific stimuli in ischemic brain tissue. These nanomedicines aim to improve drug targeting by delivering therapeutic agents directly to the affected regions while minimizing adverse effects.
Contributions and Key Findings
This study explores the limitations of conventional drug delivery methods in the treatment of IS, particularly the challenge of insufficient drug accumulation in ischemic tissues. One of the key contributions of this research is the development of stimuli-responsive nanomedicines, which can release their drug payloads precisely in response to specific endogenous and exogenous stimuli. These stimuli-responsive systems offer more effective targeting of therapeutic drugs, ensuring that they are delivered directly to the ischemic regions while reducing the risk of side effects. Below are the major findings of this research:
- ROS-Responsive Nanomedicines: These nanomedicines are designed to respond to elevated levels of reactive oxygen species (ROS), which are present in ischemic brain tissues. The study showed that ROS-responsive nanomedicines were effective in reducing infarct volume and improving neurological outcomes in animal models. By utilizing ROS as a trigger for drug release, these nanomedicines help in delivering therapeutic agents directly to the ischemic areas, thus promoting tissue repair and protecting brain cells from further damage.
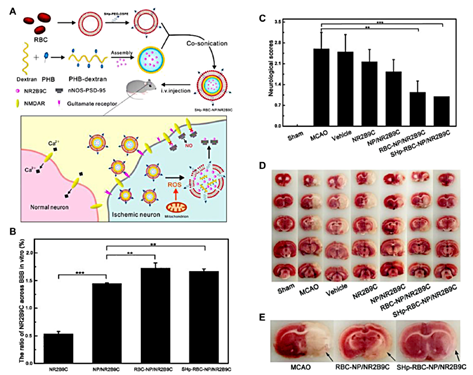
Figure 1: The fabrication of SHp-RBC-NP/NR2B9C and the results of animal experiments.
- pH-Responsive Nanomedicines: The ischemic brain microenvironment is typically more acidic due to metabolic changes such as anaerobic glycolysis. This study developed pH-responsive nanomedicines that can release drugs in response to the acidic conditions found in ischemic tissues. These nanomedicines have shown improved drug delivery efficiency at the site of injury, enhancing therapeutic efficacy by exploiting the unique pH alterations in ischemic tissues.
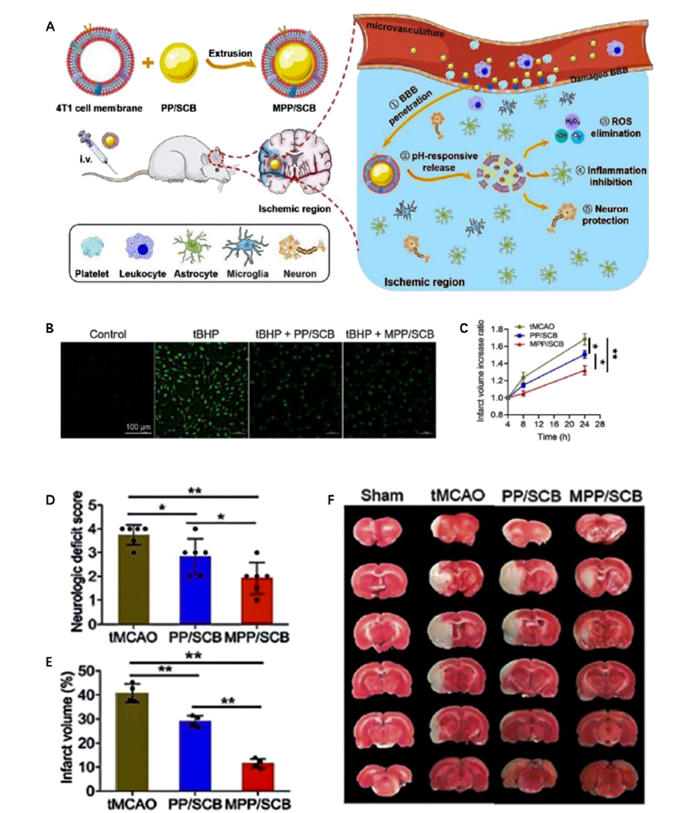
Figure 2: The preparation and therapeutic effect of pH-responsive MPP/SCB nanomedicine.
- Exogenous Stimuli: The research also explored the potential of magnetic fields and light as exogenous stimuli to enhance drug targeting. Magnetic-responsive nanomedicines, when exposed to external magnetic fields, can accumulate in the ischemic brain regions, allowing for targeted drug delivery. Additionally, light-responsive nanomedicines, which release drugs upon exposure to specific light wavelengths, offer a precise control mechanism for drug release. These systems enhance the targeted delivery of drugs, thereby improving treatment outcomes and minimizing the risk of off-target effects.
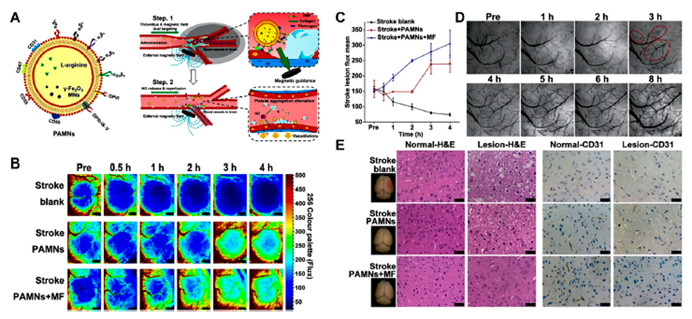
Figure 3: Magnetic stimuli-responsive platelet membrane biomimetic nanomedicine for anti-IS therapy.
Methodology and Applications
The study synthesizes findings from both in vivo and in vitro experiments to design nanomedicines that can respond to biochemical changes in ischemic tissues. The research focuses on the design of nanomedicines that are responsive to ROS production, pH changes, and other biological signals specific to the ischemic environment. Additionally, the study also investigates the use of magnetic fields, light, and ultrasound as external triggers for enhancing drug targeting.
A primary aspect of the methodology involves engineering nanomedicines with properties that not only facilitate targeted drug release but also mitigate the adverse effects commonly associated with traditional stroke treatments. This includes reducing systemic toxicity, minimizing the risk of brain hemorrhage, and ensuring drugs are delivered only to the ischemic brain regions, thus enhancing the safety profile of the treatment.
Impact on Stroke Therapy
The findings from this study have significant implications for the treatment of ischemic stroke. The research demonstrates the potential of stimuli-responsive nanomedicines to address the limitations of current drug delivery methods, particularly the challenge of ensuring that drugs reach the ischemic areas effectively. By using advanced targeting mechanisms that respond to the unique conditions of ischemic brain tissue, these nanomedicines offer an innovative solution to improve stroke therapy outcomes.
The application of these nanomedicines not only increases the therapeutic efficacy of the drugs but also reduces the potential side effects, such as cerebral hemorrhage, that are commonly associated with traditional treatments. This targeted approach provides a more controlled and effective method for treating ischemic stroke, which could improve patient outcomes and reduce the long-term complications associated with this condition.
Conclusion
This research highlights the potential of stimuli-responsive nanomedicines to transform the treatment landscape for ischemic stroke. By utilizing specific biological signals to control the release of drugs, these nanomedicines provide a highly targeted approach that could significantly improve the efficacy of stroke treatments while minimizing adverse side effects. Although the preclinical findings are promising, further research, particularly large-scale clinical trials, is needed to validate the safety and efficacy of these nanomedicines in human populations.
The study paves the way for more effective and safer treatments for ischemic stroke, offering hope for better recovery and quality of life for patients. While challenges remain in translating these preclinical findings into clinical practice, the research marks a significant step toward more precise and tailored treatments for ischemic stroke, with the potential to improve outcomes for millions of patients worldwide.
Reference
Zhan, Yongyi, et al. “Application of Stimuli-Responsive Nanomedicines for the Treatment of Ischemic Stroke.” Frontiers in Bioengineering and Biotechnology, vol. 11, 2024, article 1329959, https://doi.org/10.3389/fbioe.2023.1329959.
