A recent study shows that P-selectin-targeted nanocarriers can effectively cross the blood-brain barrier to deliver the cancer drug vismodegib directly to medulloblastoma tumors, potentially reducing harmful side effects.
Key Highlights:
- Research Question:
Can P-selectin-targeted nanocarriers enhance the delivery of vismodegib across the blood-brain barrier directly to medulloblastoma tumors? - Research Difficulties: Delivering therapeutics across the blood-brain barrier has long posed a significant challenge due to its selective permeability, which limits drug uptake in brain tissues.
- Key Findings: The study demonstrated that fucoidan-based nanoparticles targeting endothelial P-selectin facilitated caveolin-1-dependent transcytosis, achieving effective drug delivery to tumor sites while reducing toxicity to healthy brain and bone tissues.
- Innovative Aspects: This research introduces a novel mechanism utilizing P-selectin as a target for active transport of nanoparticles across the blood-brain barrier, significantly improving drug localization in tumor areas.
- Importance of the Study: The findings offer a promising new strategy for treating medulloblastoma and potentially other central nervous system disorders, addressing both therapeutic efficacy and safety concerns associated with current treatments.
Context and Significance
Medulloblastoma is recognized as the most common malignant brain tumor in children, typically originating in the cerebellum. It is characterized by aggressive growth and a prevalence of approximately 30% attributed to Sonic Hedgehog (SHH) signaling pathways. Patients diagnosed with the SHH subgroup of medulloblastoma often experience poorer clinical outcomes due to the tumor’s ability to maintain an intact blood-brain barrier (BBB). This barrier significantly limits the delivery of therapeutic agents, such as vismodegib, which is used to inhibit the SHH pathway effector Smoothened. While effective in curtailing tumor growth, vismodegib necessitates high doses that can lead to severe side effects, including growth plate fusion in pediatric patients. The challenge of delivering therapeutics across the BBB arises from its selective permeability, which serves to protect the central nervous system but also complicates effective treatment for brain tumors.
Research Aim & Objectives
The research team, including Dr. Daniel E. Tylawsky and Dr. Hiroto Kiguchi from Memorial Sloan Kettering Cancer Center, published their findings in Nature Materials.
This study aims to develop a novel therapeutic strategy that enhances drug delivery to medulloblastoma tumors while minimizing systemic toxicity. By utilizing P-selectin-targeted nanocarriers, the researchers sought to facilitate the active transport of vismodegib across the BBB, thereby improving its localization and efficacy in treating Sonic Hedgehog subgroup medulloblastoma. The specific objectives included:
- Investigating the expression of P-selectin in the tumor vasculature and its potential as a target for drug delivery.
- Assessing the efficacy of fucoidan-based nanoparticles in encapsulating and delivering vismodegib directly to brain tumor sites.
- Evaluating the impact of low-dose radiation therapy on enhancing P-selectin expression and subsequent nanoparticle uptake.
Experimental Approach and Results
Experimental Process Outline:
- Characterization of the BBB in SHH-MB GEM models.
- Assessment of P-selectin expression in tumor vasculature before and after irradiation.
- Synthesis of fucoidan-based nanoparticles encapsulating vismodegib (FiVis).
- In vitro and in vivo testing of FiVis nanoparticles for their ability to cross the BBB.
- Evaluation of therapeutic efficacy in SHH-MB mouse models.
- Measurement of vismodegib concentration in tumor and normal brain tissue.
- Assessment of bone toxicity related to vismodegib and comparison with FiVis.
Key Experiments Introduction:
- Characterization of the BBB
Before initiating drug delivery experiments, the researchers established the integrity of the BBB in a genetically engineered mouse model of SHH-MB. They injected symptomatic mice with tetramethylrhodamine (TMR)-dextran and observed minimal extravasation into the brain tumor tissue, indicating that the BBB remained intact even at advanced stages of the disease. - P-selectin Expression Analysis
The researchers performed immunofluorescence staining to assess P-selectin expression in SHH-MB tumor vasculature. They conducted experiments at various radiation doses and found that a single dose of 0.25 Gy XRT induced robust elevation of P-selectin expression, which was confirmed by quantifying fluorescence intensity (P-selectin expression increased significantly compared to controls, *P < 0.05).
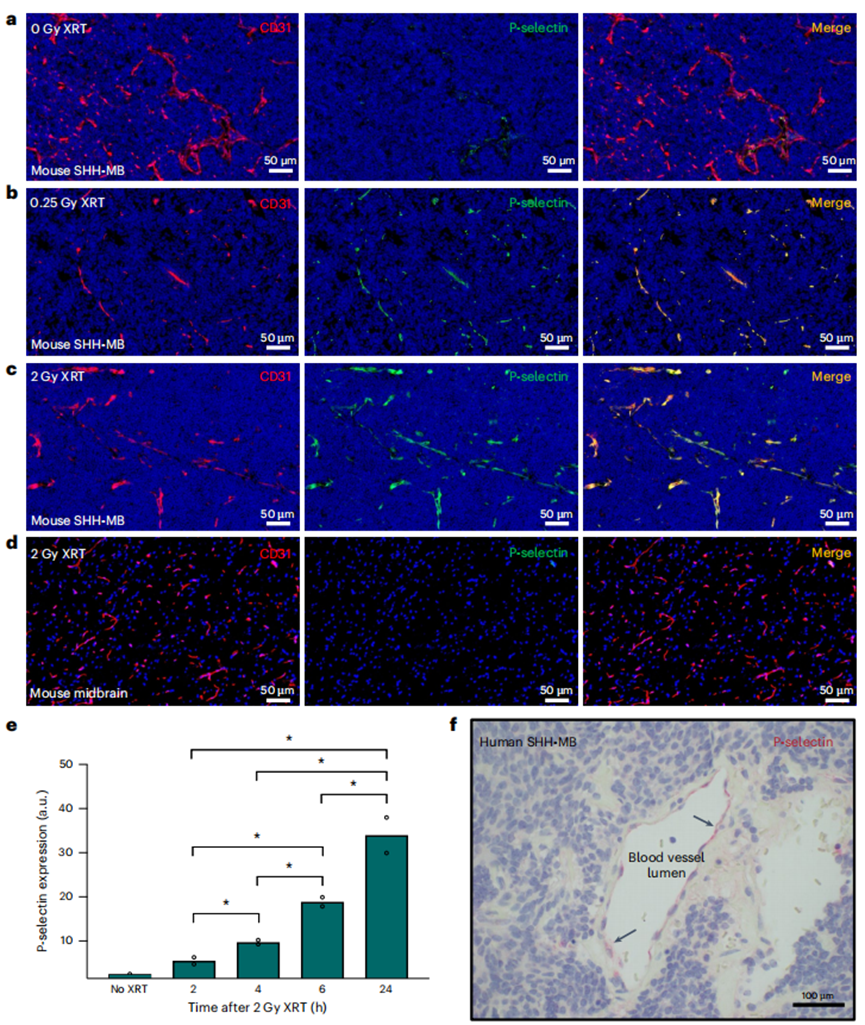
Figure 1. Low-dose irradiation induces P-selectin expression on tumour vasculature in medulloblastoma.
- Synthesis of Fucoidan-based Nanoparticles
Fucoidan-encapsulated vismodegib nanoparticles were synthesized using a nanoprecipitation method. The resulting FiVis nanoparticles exhibited an average size of 80 ± 10 nm and had a high encapsulation efficiency for vismodegib, as confirmed by HPLC analysis.
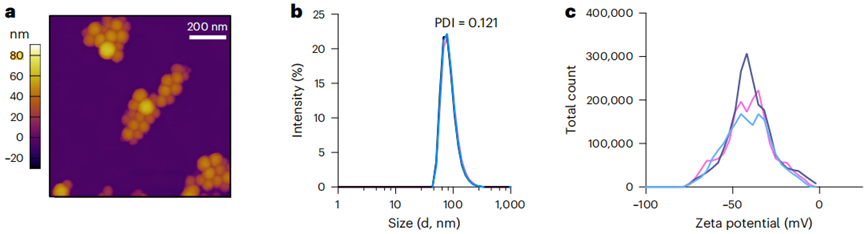
Figure 2. P-selectin-targeted nanoparticles preferentially target SHH-MB tumours following low-dose irradiation.
- In Vivo Testing of FiVis Nanoparticles
The efficacy of FiVis nanoparticles was evaluated in SHH-MB mouse models. Following irradiation, the researchers administered FiVis and measured its localization in tumor versus normal brain tissue using near-infrared imaging. The results demonstrated significant accumulation of nanoparticles in cerebellar tumor regions compared to adjacent forebrain areas (quantified fluorescence intensity in tumor regions was statistically significant, *P < 0.05). - Therapeutic Efficacy Measurement
The study used RT-qPCR to measure Gli1, a downstream effector of SHH signaling, as a marker for therapeutic efficacy. Treatment with FiVis nanoparticles resulted in approximately 80% Gli1 inhibition at a dose of 10 mg/kg, compared to higher doses of free vismodegib required to achieve similar results, illustrating the enhanced efficacy of targeted delivery.
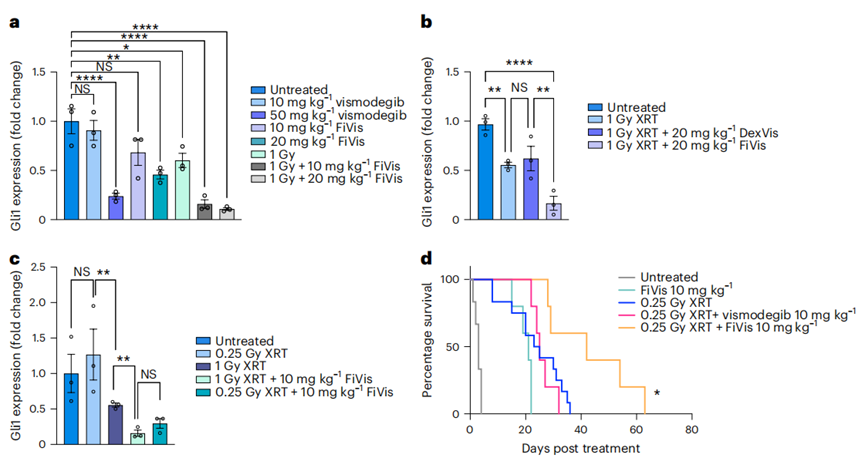
Figure 3. FiVis nanoparticles synergize with low-dose irradiation to enhance Gli1 target inhibition and survival in SHH-MB.
- Bone Toxicity Assessment
Bone toxicity related to vismodegib was assessed in juvenile mice. The study revealed that high doses of free vismodegib resulted in growth stunting and femur length abnormalities, whereas treatment with FiVis nanoparticles at equivalent therapeutic levels showed no significant adverse effects on bone growth or morphology.
Key Insights and Implications
The study presents a significant advancement in the targeted delivery of therapeutics for medulloblastoma treatment through the use of P-selectin-targeted nanocarriers. By successfully demonstrating that fucoidan-based nanoparticles can actively cross an intact blood-brain barrier and deliver vismodegib directly to tumor sites, this research highlights an innovative approach to enhance drug localization while minimizing systemic toxicity. The findings not only improve the therapeutic index of vismodegib in SHH-MB but also establish a framework for the application of targeted nanomedicine in treating other central nervous system disorders.
The researchers underscore the importance of their findings in overcoming the challenges presented by the BBB in delivering therapeutics to brain tumors. The study indicates that P-selectin targeting may extend beyond medulloblastoma to other intracranial tumors and central nervous system disorders, where P-selectin expression is upregulated. The potential to utilize this targeted delivery system for various therapeutic agents could lead to improved treatment outcomes while reducing severe side effects, paving the way for future advancements in nanomedicine and cancer therapy.
Reference:
Tylawsky, Daniel E., et al. “P-selectin-targeted nanocarriers induce active crossing of the blood–brain barrier via caveolin-1-dependent transcytosis.” Nature materials 22.3 (2023): 391-399.
