Author: Tiffany
A recent study has indicated that exosomes derived from human mesenchymal stem cells could present a new therapeutic option for treating Polycystic Ovary Syndrome (PCOS), a condition that impacts millions of women globally.
Key Highlights:
- Research Question:
Can mesenchymal stem cell-derived extracellular vesicles (exosomes) effectively treat PCOS in both in vitro and in vivo models? - Research Difficulties:
Previous studies did not effectively isolate the therapeutic effects of exosomes or explore optimal administration routes in PCOS treatment. - Key Findings:
MSC-derived exosomes significantly reduced androgen-producing gene expression, improved metabolic profiles, and restored ovarian function in PCOS mouse models. - Innovative Aspects:
This study uniquely demonstrates the effectiveness of exosome therapy compared to whole stem cell treatments and assesses both intravenous and intraovarian injection routes. - Importance of the Study:
This research provides a promising new avenue for PCOS treatment, highlighting the potential for exosomes as safer, more accessible therapeutic agents.
Background
Polycystic Ovary Syndrome (PCOS) is a prevalent endocrine disorder affecting approximately 15-18% of women of reproductive age globally. Characterized by hyperandrogenism, ovulatory dysfunction, and polycystic ovarian morphology, PCOS can lead to various complications, including infertility, metabolic syndrome, and increased cardiovascular risks. Women with PCOS often experience insulin resistance, dyslipidemia, and obesity, exacerbating their health conditions. Despite the high prevalence of PCOS, the pathogenesis remains unclear, involving a complex interplay of genetic, environmental, and lifestyle factors. Current therapeutic strategies primarily focus on symptom management rather than addressing the underlying causes of the disorder. Treatments such as hormonal contraceptives, insulin sensitizers, and fertility medications are commonly prescribed, but they may not yield satisfactory results for all patients. Recent advances in regenerative medicine, particularly the use of mesenchymal stem cells (MSCs) and their secreted factors, have shown promise in addressing the inflammation and hormonal dysregulation associated with PCOS. However, the specific therapeutic potential of MSC-derived exosomes in treating PCOS has not been thoroughly investigated.
Research Aim & Objectives
This study aims to evaluate the therapeutic potential of extracellular vesicles (exosomes) derived from human mesenchymal stem cells (MSCs) in the treatment of PCOS. The primary objectives include:
- Assessing whether MSC-derived exosomes can effectively reduce androgen production in vitro using human adrenocortical carcinoma (H295R) cells.
- Investigating the in vivo effects of MSC-derived exosomes on metabolic profiles, ovarian function, and fertility in letrozole-induced PCOS mouse models.
- Comparing the efficacy of exosome administration via different injection routes—intravenous (IV) and intraovarian (IO)—to determine the optimal delivery method for therapeutic benefits.
Research Methods & Results
Experimental Process Outline
- Preparation of H295R Cell Culture:
- Use androgen-producing H295R cells for in vitro studies.
- Isolation of MSC-Derived Exosomes:
- Collect conditioned media from human MSC cultures.
- Isolate exosomes using PEG-precipitation method.
- Treatment of H295R Cells:
- Treat H295R cells with MSC-derived conditioned media and exosomes for 24 hours.
- Gene Expression Analysis:
- Perform qRT-PCR to evaluate changes in androgen-producing gene expression (Cyp17a1, Cyp11a1, DENND1a).
- PCOS Mouse Model Induction:
- Implant letrozole (LTZ) pellets in female C57BL/6 mice to induce PCOS.
- Exosome Treatment:
- Administer MSC-derived exosomes via IV and IO injection.
- Assessment of Metabolic Parameters:
- Measure body weight changes and adipocyte sizes in white adipose tissue. – Conduct glucose tolerance tests (GTT).
- Evaluation of Ovarian Function:
- Analyze estrous cycle patterns and serum hormone levels. – Perform histological analysis of ovarian tissues.
- Breeding Experiments:
- Evaluate fertility outcomes by assessing pregnancy rates and litter sizes.
Key Experiments
- Effect of MSC-Derived Exosomes on Androgen-Producing Cells:
- Preparatory Work: H295R cells were cultured and treated with MSC-derived conditioned media (MSC CM) and isolated exosomes.
- Procedure: H295R cells were treated for 24 hours, and gene expression levels of androgen-producing genes (Cyp17a1, Cyp11a1, DENND1a) were analyzed using quantitative real-time polymerase chain reaction (qRT-PCR).
- Results: Cyp17a1 expression decreased significantly in MSC CM (0.69 ± 0.02-fold) and exosome-treated cells (0.58 ± 0.04-fold) compared to the control (p = 0.16). Cyp11a1 expression was also significantly reduced in MSC CM (0.82 ± 0.08-fold) and exosome-treated cells (0.75 ± 0.03-fold) without significant difference (p = 0.37). DENND1a expression decreased in both treatment groups (MSC CM: 0.71 ± 0.02-fold; exosome: 0.72 ± 0.06-fold).
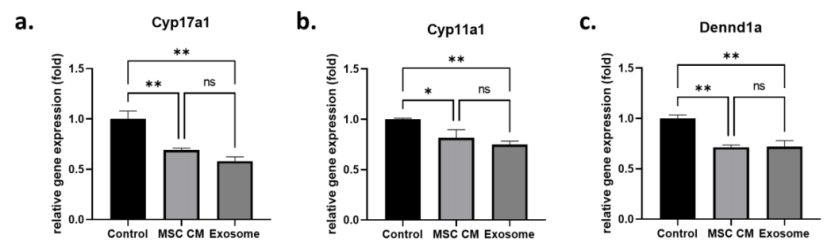
Figure 1. Effect of MSC-derived exosomes on androgen production in an in vitro cell model.
- MSC-Derived Exosome Treatment Reverses Metabolism in a PCOS Mouse Model:
- Preparatory Work: PCOS was induced in female C57BL/6 mice using LTZ pellets.
- Procedure: Mice were treated with MSC-derived exosomes through IV and IO injection, and body weight, adipocyte size, and glucose tolerance were assessed.
- Results: The body weight of the PCOS group was significantly higher (21.0 ± 0.0 g) compared to exosome-IV (19.7 ± 0.6 g) and exosome-IO (20.0 ± 0.0 g). Adipocyte size in the exosome-IV group decreased to 5198 ± 3306 µm² and in the exosome-IO group to 4424 ± 2660 µm² compared to the untreated PCOS group (9030 ± 5797 µm²). In glucose tolerance tests, blood glucose levels were significantly lower in the exosome-IV group, mirroring healthy control trends.
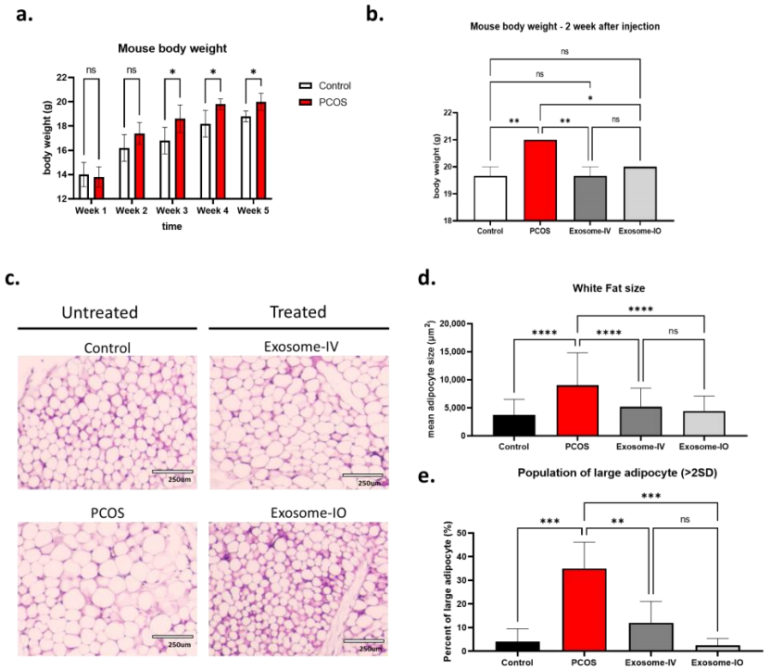
Figure 2. Regulation of metabolic parameters by MSC-derived exosome treatment in a PCOS mouse model.
- MSC-Derived Exosomes Restore Ovarian Function in a PCOS Mouse Model:
- Preparatory Work: Estrous cycle monitoring and serum hormone analysis were conducted.
- Procedure: Estrous cycles were recorded, and serum levels of testosterone, estradiol, FSH, and LH were measured post-treatment.
- Results: The PCOS group exhibited high testosterone levels (63.0 ± 12.9 ng/dL), which were reduced in both exosome-IV (45.1 ± 1.7 ng/dL) and exosome-IO (31.6 ± 8.6 ng/dL). LH levels were significantly higher in the PCOS group (1.16 ± 0.62 ng/mL) compared to controls and were reduced in both treatment groups (exosome-IV: 0.49 ± 0.10 ng/mL; exosome-IO: 0.41 ± 0.1 ng/mL). Histological analysis revealed more follicles and reduced cystic morphology in exosome-treated ovaries.
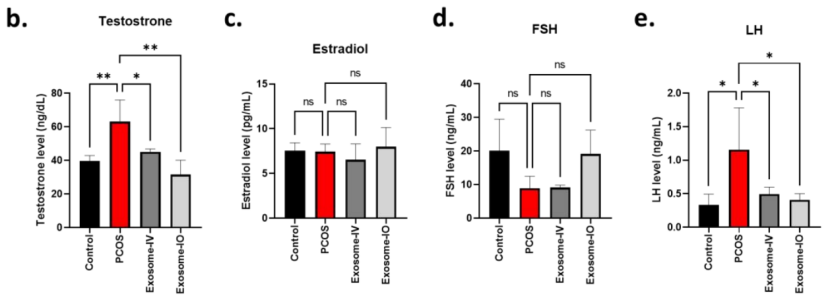
Figure 3. Serum hormone level comparison between the control, PCOS, exosome-IV, and exosome-IO groups (n = 3/group).
- MSC-Derived Exosomes Restore Fertility in a PCOS Mouse Model:
- Preparatory Work: Breeding experiments were set up with treated and control mice.
- Procedure: Female mice were bred with a male, and pregnancy rates and litter sizes were recorded.
- Results: The pregnancy rate in the PCOS group was only 25%, while both exosome-IV (50%) and exosome-IO (100%) groups showed restored fertility. Average litter sizes were significantly improved in the exosome-IV (9.5 ± 0.7 pups) and exosome-IO (6.8 ± 2.2 pups) groups compared to the untreated PCOS group (1 pup).
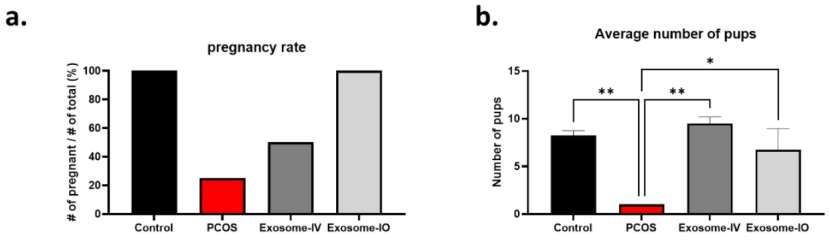
Figure 4. Fertility restoration by MSC-derived exosomes in a PCOS mouse model.
Summary
This study demonstrates that MSC-derived exosomes exhibit significant therapeutic potential in reversing the conditions associated with PCOS. The research innovatively highlights the ability of these exosomes to reduce androgen production and restore both metabolic function and fertility in PCOS mouse models. Notably, the findings reveal that intravenous injection is more effective for systemic metabolic regulation, while intraovarian injection more effectively restores ovarian function and fertility. The researchers reflect on the implications of their research, emphasizing the promise of exosome-based therapies as a novel treatment approach for PCOS. They acknowledge the need for further validation through clinical trials to assess the safety and efficacy of MSC-derived exosomes in human patients. Future studies are planned to initiate clinical trials targeting PCOS treatment with MSC-derived exosomes, aiming to provide a safer, more accessible therapeutic option for women suffering from this complex endocrine disorder.
Reference:
Park, Hang-Soo, et al. “Therapeutic potential of mesenchymal stem cell-derived extracellular vesicles to treat PCOS.” International Journal of Molecular Sciences 24.13 (2023): 11151.
