Author: Tiffany
A recent study has developed a tumor-specific gene nanomedicine that enhances T-cell recruitment and activation, leading to improved effectiveness in the treatment of melanoma through immunotherapy.
Key Highlights:
- Research Question:
Can tumor-specific gene nanomedicines effectively enhance T-cell recruitment and activation in melanoma, thereby improving cancer immunotherapy outcomes? - Research Difficulties:
Achieving sufficient T-cell infiltration and high concentrations of PD-1/PD-L1 inhibitors within tumors has been challenging, leading to poor responses to existing immunotherapies. - Key Findings:
The new nanomedicine, driven by a tyrosinase promoter, enables melanoma cells to secrete CXCL9 and an anti-PD-L1 agent, resulting in robust T-cell recruitment and enhanced anti-tumor effects. - Innovative Aspects:
This study is unique in using a dual-action nanomedicine that simultaneously promotes T-cell recruitment and blocks PD-L1, addressing two critical barriers in melanoma treatment. - Importance of the Study:
The findings provide a promising strategy for overcoming limitations of current immunotherapies, potentially leading to more effective treatments for melanoma and other cancers.
Background on Melanoma
Melanoma is a highly aggressive form of skin cancer that originates from melanocytes, the pigment-producing cells in the skin. This cancer is particularly concerning due to its high propensity for metastasis, often spreading to other organs and tissues, which complicates treatment options. Melanoma can manifest as changes in existing moles or the emergence of new skin lesions, with symptoms including irregularly shaped moles, changes in pigmentation, and sores that do not heal. Early detection is crucial, as the prognosis is significantly better when the disease is diagnosed at an early stage. Current therapeutic strategies for melanoma include surgical intervention, targeted therapies, and immunotherapy. Among these, immune checkpoint inhibitors, such as PD-1 and PD-L1 blockers, have revolutionized the treatment landscape by unleashing the immune system’s ability to recognize and attack cancer cells.
However, despite these advancements, the response rates remain suboptimal, with many patients experiencing limited benefits due to inadequate T-cell infiltration within tumors and the insufficient concentration of therapeutic agents in the tumor microenvironment. This highlights the need for more effective treatment modalities that can enhance T-cell recruitment and activation within tumors, thereby improving patient outcomes.
Research Aim & Objectives
Conducted by researchers at the South China University of Technology, the study was published in Nature Communications on March 27, 2023. The primary aim of this study is to investigate the potential of a novel tumor-specific gene nanomedicine, NPTyr-C9AP, to enhance T-cell recruitment and activation in melanoma. Specifically, the study seeks to address the challenges associated with current immunotherapies, particularly the limitations in T-cell infiltration and the effectiveness of PD-1/PD-L1 blockade.
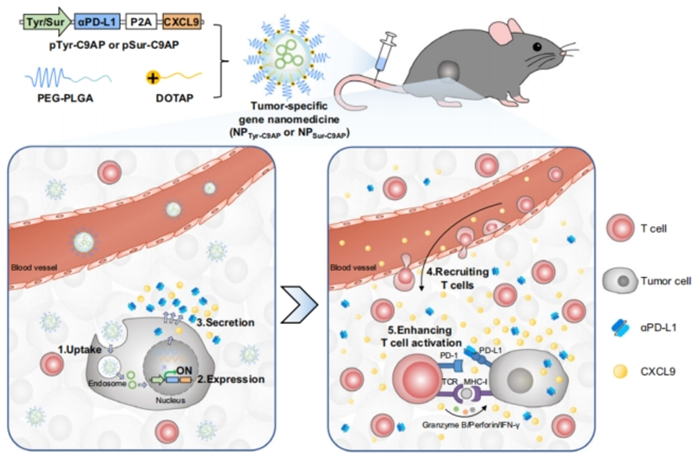
Figure 1. Schematic illustration of devising tumor-specific gene nanomedicines to recruit and activate T cells for enhanced immunotherapy.
Research Objectives:
- Development of NPTyr-C9AP: To construct a co-expression plasmid that utilizes the tyrosinase promoter to drive the expression of CXCL9 and αPD-L1 specifically in melanoma cells. This involves engineering biodegradable nanoparticles for efficient gene delivery.
- Evaluation of Specificity and Efficacy: To assess the melanoma specificity of NPTyr-C9AP and its ability to induce the secretion of CXCL9 and αPD-L1 in melanoma cells. This objective includes conducting in vitro experiments to quantify the levels of T-cell recruitment and activation.
- Investigation of Mechanisms: To explore the mechanisms by which NPTyr-C9AP enhances T-cell recruitment and activation, including the establishment of a CXCL9 gradient that guides T-cells to the tumor site and the blockade of PD-L1 that prevents T-cell exhaustion.
- In vivo Therapeutic Assessment: To evaluate the therapeutic efficacy of NPTyr-C9AP in preclinical mouse models of melanoma, focusing on tumor growth inhibition, survival rates, and the analysis of tumor-infiltrating T cells.
- Comparison with Existing Therapies: To compare the performance of NPTyr-C9AP with traditional immunotherapies, notably systemic PD-L1 antibodies, to determine its advantages in terms of efficacy and safety. Through these objectives, the study aims to establish a promising new strategy for melanoma treatment that could ultimately lead to more effective immunotherapeutic approaches in clinical settings.
Research Methods & Results
Experimental Process Outline
- Construction of a co-expression plasmid (pTyr-C9AP) using the tyrosinase promoter.
- Encapsulation of the plasmid into biodegradable nanoparticles to create NPTyr-C9AP.
- Characterization of NPTyr-C9AP, including hydrodynamic diameter, zeta potential, and morphology.
- Assessment of melanoma specificity by transfecting various cell lines with NPTyr-C9AP.
- Evaluation of CXCL9 and αPD-L1 expression in transfected melanoma cells.
- In vitro analysis of T-cell recruitment using transwell migration assays.
- Measurement of cytotoxicity enhancement via co-culture of T cells and melanoma cells.
- In vivo evaluation of therapeutic efficacy in mouse models of melanoma, including monitoring tumor growth and survival.
- Analysis of tumor-infiltrating T cells and their activation status post-treatment.
- Comparison of NPTyr-C9AP with other treatment modalities, including anti-PD-L1 antibodies.
Key Experiments
- Construction and Characterization of NPTyr-C9AP:
- Preparatory Work: The plasmid pTyr-C9AP was constructed to enable the melanoma-specific co-expression of CXCL9 and αPD-L1. This was achieved by inserting the CXCL9 and αPD-L1 sequences downstream of the tyrosinase promoter in a pUC57 backbone plasmid.
- Experimental Procedure: NPTyr-C9AP was synthesized by encapsulating the constructed plasmid into nanoparticles composed of PEG-PLGA and DOTAP using the double emulsion method. The size and zeta potential of NPTyr-C9AP were analyzed using dynamic light scattering (DLS) and transmission electron microscopy (TEM).
- Results: The hydrodynamic diameter of NPTyr-C9AP was found to be 107.2 nm with a zeta potential of 15.3 mV, indicating favorable properties for tumor accumulation via the enhanced permeability and retention (EPR) effect. TEM images confirmed the spherical shape of the nanoparticles, which remained stable over five days in the physiological environment.
- New Finding: These findings establish that NPTyr-C9AP possesses the desired characteristics for effective delivery of the therapeutic genes specifically to melanoma cells.
- Assessment of Melanoma Specificity:
- Preparatory Work: Various cell lines, including B16-F10 melanoma cells, CT26, Panc02, among others, were prepared for transfection with NPTyr-C9AP.
- Experimental Procedure: The cell lines were exposed to Cy5-labeled NPTyr-C9AP for 4 hours. After incubation, the internalization of NPTyr-C9AP was monitored using confocal laser scanning microscopy (CLSM) and flow cytometry.
- Results: CLSM imaging revealed stronger Cy5 fluorescence in B16-F10 cells compared to other cell types, indicating efficient internalization. Flow cytometry confirmed nearly 100% uptake across all eight cell types, but the expression of CXCL9 and αPD-L1 mRNA was significantly higher in B16-F10 cells, achieving 586.4-fold and 1554.0-fold increases, respectively.
- New Finding: This experiment demonstrates that NPTyr-C9AP can specifically target melanoma cells for effective gene delivery, significantly enhancing the potential for tailored melanoma therapy.
- T-cell Recruitment Analysis:
- Preparatory Work: After confirming the expression of CXCL9 in B16-F10 cells, a transwell migration assay was designed to evaluate the recruitment of activated CD8+ T cells.
- Experimental Procedure: Supernatants from B16-F10 cells transfected with PBS, NPControl, NPTyr-CXCL9, or NPTyr-C9AP were placed in the lower chamber of transwell plates, while DiI-labeled CD8+ T cells were added to the upper chamber. The T-cell migration was quantified at various time points.
- Results: The supernatants from NPTyr-CXCL9 and NPTyr-C9AP-transfected B16-F10 cells recruited significantly more CD8+ T cells, with counts of 1.26 × 10^5 and 1.14 × 10^5 cells, respectively, at the 180-minute mark.
- New Finding: This experiment confirms that the CXCL9 secreted by melanoma cells effectively establishes a chemotactic gradient, leading to significant T-cell recruitment, which is crucial for enhancing anti-tumor immunity.
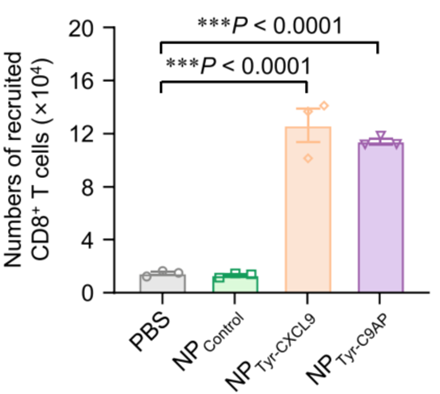
Figure 2. CLSM imaging of CD8+ T cells (stained with DiI dye, red) recruited into the lower chamber of the transwell plates after addition of the culture supernatants of the B16-F10 cells transfected with NPTyr-C9AP or other controls.
- Cytotoxicity Enhancement Analysis:
- Preparatory Work: B16-F10-OVA-EGFP cells were stimulated with IFN-γ to increase PD-L1 expression, and then co-cultured with OVA-specific CD8+ T cells.
- Experimental Procedure: The culture supernatants of B16-F10 cells transfected with PBS, NPControl, NPTyr-αPD-L1, or NPTyr-C9AP were used to assess their cytotoxic effects on the IFN-γ-stimulated B16-F10-OVA-EGFP cells via propidium iodide (PI) staining and CCK-8 assays.
- Results: The PI-positive percentages of IFN-γ-stimulated B16-F10-OVA-EGFP cells were significantly higher in the NPTyr-αPD-L1 (45.4%) and NPTyr-C9AP (42.8%) groups compared to the control groups (4.9% for PBS and NPControl). The CCK-8 assay corroborated these findings with similarly low viability percentages for the treatment groups.
- New Finding: This experiment illustrates that the combination of CXCL9 and αPD-L1 significantly enhances the cytotoxic activity of T cells against melanoma cells, providing a dual mechanism for tumor suppression.
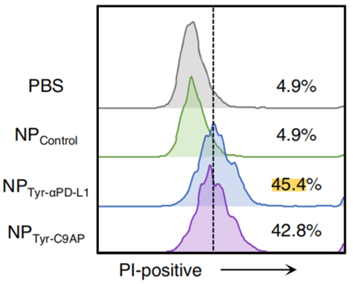
Figure 3. Flow cytometry analysis of PI-positive (dead) percentages and CCK-8 assays of cell viability.
- In vivo Therapeutic Efficacy Evaluation:
- Preparatory Work: C57BL/6 mice bearing B16-F10 melanoma were treated with NPTyr-C9AP to evaluate its therapeutic efficacy.
- Experimental Procedure: Mice were intravenously injected with NPTyr-C9AP every other day for five doses. Tumor growth was monitored, and final tumor weights were measured at the end of the treatment period.
- Results: The tumor growth inhibition rate for the NPTyr-C9AP group reached 84.3%, with final tumor weights significantly reduced to only 16.7% of the PBS group. The median survival time for NPTyr-C9AP-treated mice was also significantly extended compared to control groups.
- New Finding: This experiment demonstrates the potent therapeutic potential of NPTyr-C9AP in vivo, significantly inhibiting tumor growth and enhancing survival rates in melanoma-bearing mice.
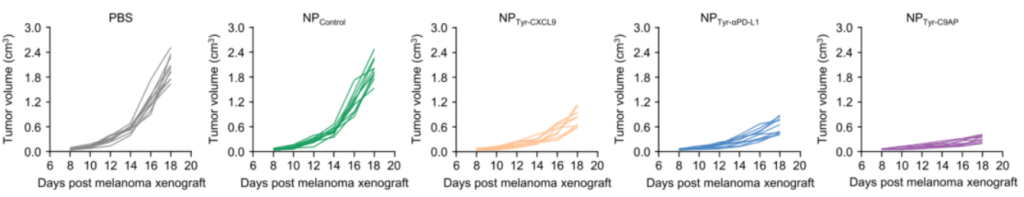
Figure 4. Individual and average tumor growth curves of the B16-F10 melanoma-bearing mice treated with NPTyr-C9AP or other controls (n = 10 mice per group).
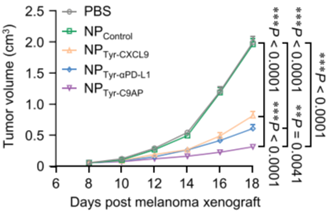
Figure 5. Survival curves of the B16-F10 melanoma-bearing mice treated with NPTyr-C9AP or other controls (n = 10 mice per group).
- Analysis of Tumor-Infiltrating T Cells:
- Preparatory Work: Post-treatment tumor tissues were collected for flow cytometry to analyze T-cell infiltration.
- Experimental Procedure: Tumor tissues from mice treated with NPTyr-C9AP or other controls were processed to assess the percentages of CD3+ and CD8+ T cells.
- Results: The NPTyr-C9AP treatment significantly increased CD3+ T cells to 21.4% of CD45+ lymphocytes, compared to 10.4% in the NPTyr-CXCL9 group and 8.9% in the NPTyr-αPD-L1 group. The number of CD69-positive CD8+ T cells also increased significantly, indicating enhanced T-cell activation.
- New Finding: This experiment highlights the synergistic effect of CXCL9 and αPD-L1 on enhancing both the quantity and activation status of tumor-infiltrating T cells, which is crucial for effective anti-tumor immunity.
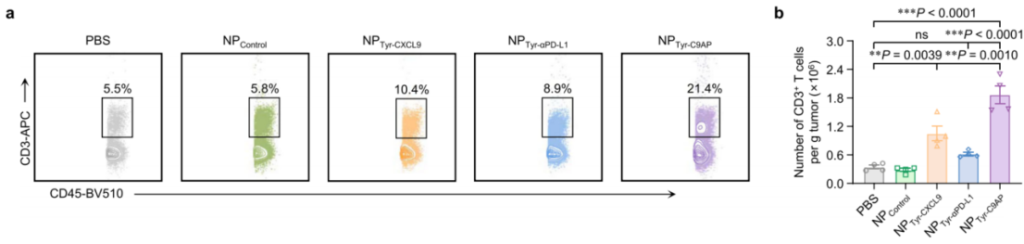
Figure 6. Flow cytometry plots of CD3+ T cell percentages in CD45+ cells or numbers of CD3+ T cells of the melanoma samples from the mice treated with NPTyr-C9AP or other controls (n = 4 mice per group).
Summary
This study presents significant advancements in melanoma treatment through the development of a tumor-specific gene nanomedicine, NPTyr-C9AP, which effectively enhances T-cell recruitment and activation. The key findings of the research include:
- Enhanced T-cell Recruitment and Activation: NPTyr-C9AP successfully induces melanoma cells to secrete CXCL9 and αPD-L1, leading to increased infiltration of T cells into the tumor microenvironment. The study demonstrated that the co-expression of these two factors significantly boosted both the quantity and cytotoxic activity of tumor-infiltrating T cells, achieving a tumor growth inhibition rate of 84.3% in vivo.
- Specificity and Efficacy: The engineered nanomedicine showed a high degree of specificity for melanoma cells, allowing for localized expression of therapeutic agents while minimizing off-target effects in normal tissues. This specificity is crucial for enhancing the therapeutic index of immunotherapy in melanoma, a cancer known for its aggressive nature and complex immune evasion mechanisms.
- Comparison with Traditional Therapies: The study highlighted that NPTyr-C9AP outperforms systemic anti-PD-L1 antibody treatments in terms of efficacy and safety, suggesting a novel approach to enhance the effectiveness of existing immunotherapies. The innovation of this research lies in its unique strategy of utilizing tumor-specific promoters to drive the expression of T-cell chemoattractants and immune checkpoint inhibitors directly within the tumor. This dual-action approach not only addresses the challenge of poor T-cell infiltration but also ensures that PD-L1 blockade occurs precisely where it is needed, thus enhancing the overall anti-tumor immune response.
The significance of this study is underscored by its potential to improve clinical outcomes for melanoma patients, particularly those who have not responded well to conventional therapies. In their reflections on the research, the authors emphasize that while the NPTyr-C9AP nanomedicine shows great promise, further investigations are necessary to fully understand its long-term effects and potential applications in other tumor types. They suggest that future studies could explore the combination of this strategy with other forms of immunotherapy, such as CAR-T cell therapy or vaccines, to further enhance anti-tumor immunity. Additionally, the authors recognize the need to investigate the safety profile of the plasmid delivery system and assess any potential immunogenicity before progressing towards clinical applications. Overall, this research opens new avenues for cancer treatment and highlights the importance of innovative therapeutic strategies in overcoming current limitations in immunotherapy.
Reference:
Wang, Yue, et al. “Engineering tumor-specific gene nanomedicine to recruit and activate T cells for enhanced immunotherapy.” Nature Communications 14.1 (2023): 1993.
