Editor: Nina
This study presents a novel combination therapy using cabazitaxel-loaded human serum albumin nanoparticles and TGFβ-1 siRNA lipid nanoparticles, demonstrating enhanced efficacy and reduced toxicity in treating paclitaxel-resistant non-small cell lung cancer.
Key Preview
Research Question:
How can a combination of cabazitaxel-loaded human serum albumin nanoparticles (CTX-HSA-NPs) and TGFβ-1 siRNA lipid nanoparticles (LNP) effectively treat paclitaxel-resistant non-small cell lung cancer (NSCLC)?
Research Design and Strategy:
The study develops a dual therapeutic approach using CTX-HSA-NPs and TGFβ-1 siRNA LNP to enhance the efficacy of treatments while minimizing toxicity.
Method:
The researchers used in vitro and in vivo methodologies to assess the antitumor effects, toxicity levels, and drug delivery efficiency of the nanoparticles.
Key Results:
CTX-HSA-NPs exhibited a superior antitumor effect compared to traditional CTX-Tween, with toxicity levels reduced by 1.8 times. The combination therapy significantly enhanced the antitumor efficacy against paclitaxel-resistant NSCLC cells.
Significance of the Research:
This study presents a promising new strategy for treating drug-resistant lung cancer, potentially leading to more effective and safer therapies.
Introduction
Lung cancer remains one of the leading causes of cancer-related deaths globally, with non-small cell lung cancer (NSCLC) constituting the majority of cases. Traditional chemotherapy, while effective, often leads to severe toxicity and drug resistance, particularly with drugs like paclitaxel. Recent advancements have highlighted the potential of combining chemotherapy with novel therapies, such as small interfering RNA (siRNA), to counteract resistance mechanisms.
This study aims to address the challenge of paclitaxel resistance in NSCLC by developing a combination therapy using cabazitaxel-loaded human serum albumin nanoparticles (CTX-HSA-NPs) and TGFβ-1 siRNA lipid nanoparticles (LNP). The hypothesis posits that this dual approach could enhance therapeutic effectiveness while reducing toxicity.
Research Team and Objective
The research was conducted by a team of scholars from Hangzhou Normal University, led by Tiantian Tan, alongside Yuxin Feng, Weimin Wang, Rongrong Wang, Liyan Yin, Yiying Zeng, Zhaowu Zeng, and Tian Xie. The findings are published in the journal Cancer Nanotechnology under the title “Cabazitaxel-loaded human serum albumin nanoparticles combined with TGFβ-1 siRNA lipid nanoparticles for the treatment of paclitaxel-resistant non-small cell lung cancer”.
Experimental Process
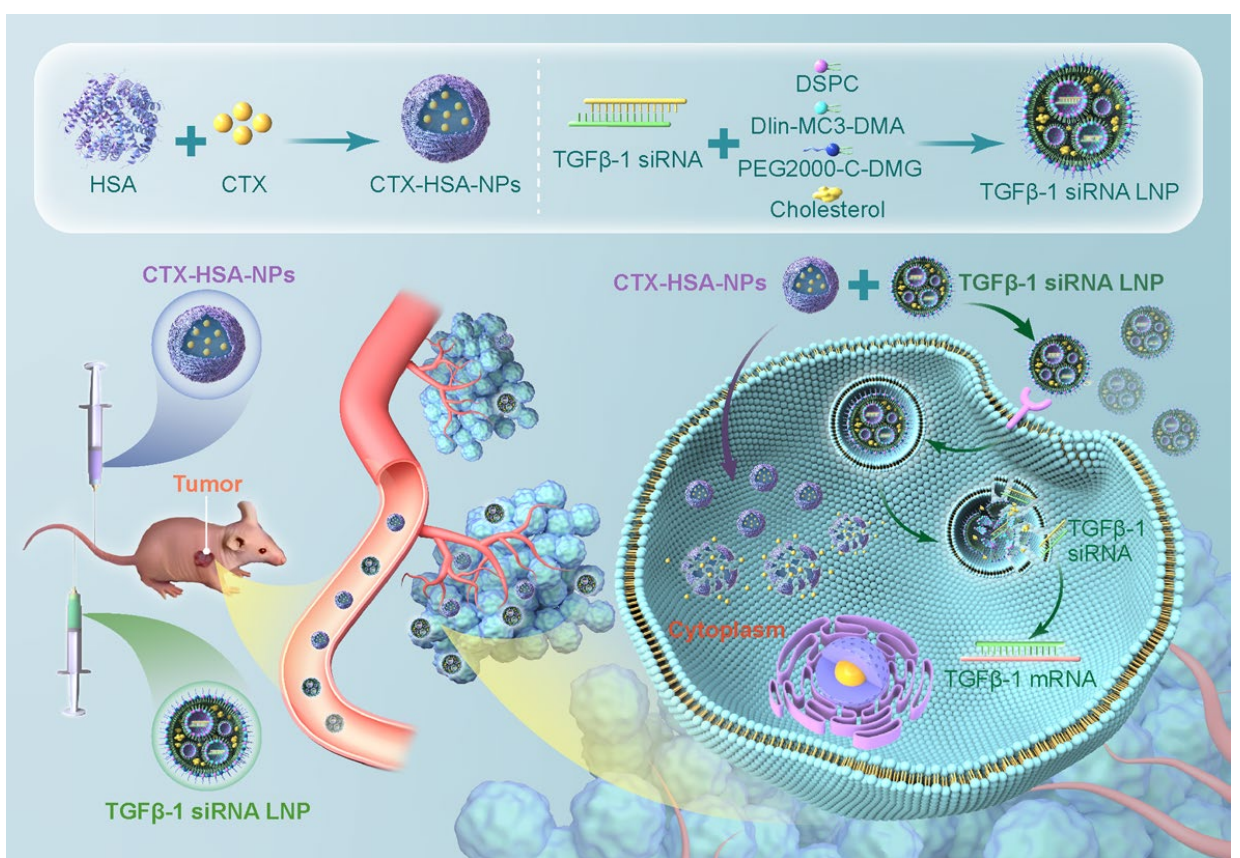
- Preparation of CTX-HSA-NPs
Key Steps:
- Human serum albumin (HSA) was dissolved in water to form the aqueous phase. Cabazitaxel (CTX) was dissolved in a mixture of chloroform and ethanol (9:1) to create the organic phase.
- The organic phase was injected into the aqueous phase and subjected to high-speed shearing to form an emulsion. – The emulsion was homogenized at 1500 bar pressure for 4 minutes using rapid cycling-cooling homogenization.
- The organic solvent was removed using a desolvation method, resulting in the formation of CTX-HSA-NPs, which were then filtered through a 0.22 μm sterile filter and lyophilized to obtain a stable powder.
Results and Key Data: - The resulting nanoparticles had a particle size of less than 120 nm, with an encapsulation efficiency (EE) of 96.82 ± 0.20%.
Significance of the Result: - The high encapsulation efficiency indicates effective loading of the chemotherapeutic agent, which is crucial for enhancing the therapeutic efficacy while minimizing systemic toxicity.
Key Innovations: - The use of HSA not only serves as a drug carrier but also enhances the biocompatibility and bioavailability of CTX, thus potentially improving its therapeutic profile compared to traditional formulations.
- Characterization of CTX-HSA-NPs
Key Steps:
- The size and polydispersity index (PDI) of CTX-HSA-NPs were measured using dynamic light scattering (DLS).
- The morphology of the nanoparticles was examined using transmission electron microscopy (TEM).
- Zeta potential was assessed to evaluate the surface charge, which influences stability and cellular uptake.
Results and Key Data: - The nanoparticle characteristics included a PDI of less than 0.3, indicating a uniform size distribution, and a zeta potential of around +0.18 mV, suggesting good stability in physiological conditions.
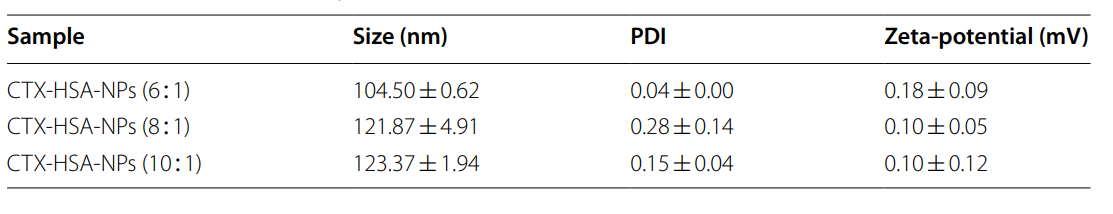
Table 1. Particle size and zeta-potential of CTX-HSA-NPs (n = 3)
Significance of the Result:
- Uniform size distribution and stability are essential for consistent drug delivery and therapeutic outcomes.
Key Innovations: - The combination of microfluidic self-assembly techniques with lyophilization allows for the generation of nanoparticles with enhanced stability and ease of reconstitution for clinical use.
- Preparation of TGFβ-1 siRNA LNP
Key Steps:
- A microfluidic chip was employed to prepare TGFβ-1 siRNA LNP. The aqueous phase contained siRNA dissolved in an acetic acid-sodium acetate buffer, while the organic phase comprised a mixture of lipids including 1,2-Dioctadecanoyl-sn-glycero-3-phosphocholine (DSPC), DLin-MC3-DMA, cholesterol, and PEG2000-c-DMG.
- The two phases were rapidly mixed in a 1:3 ratio using the microfluidic chip, followed by ultrafiltration to remove any unencapsulated siRNA.
Results and Key Data: - The average particle size of the prepared TGFβ-1 siRNA LNP was approximately 48.1 ± 0.9 nm, with an encapsulation efficiency of 97.05 ± 1.12%.
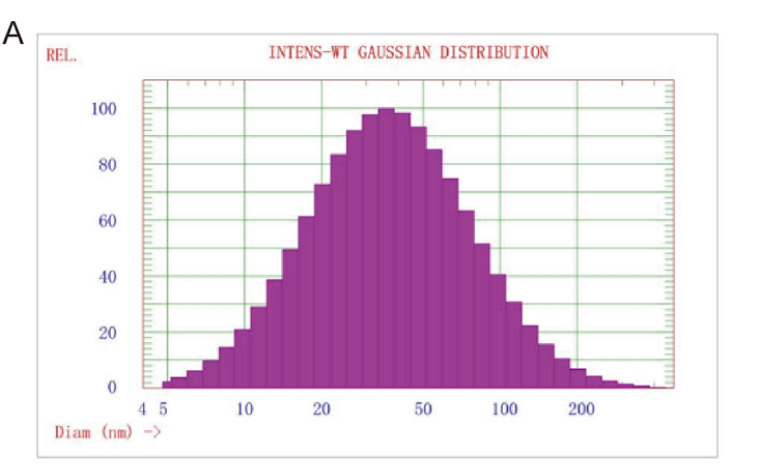
Figure 1. Particle size of TGFβ-1 siRNA LNP
Significance of the Result:
- The high encapsulation efficiency and small size of the LNP are critical for effective cellular uptake and gene silencing capability, which is vital for overcoming drug resistance.
Key Innovations: - The use of a microfluidic system for LNP preparation allows for precise control over nanoparticle characteristics, improving reproducibility and scalability for clinical applications.
- In Vitro Antitumor Activity Assessment
Key Steps:
- A549/T paclitaxel-resistant NSCLC cells were treated with various formulations: TGFβ-1 siRNA LNP, CTX-HSA-NPs, and their combination. – Cell viability was measured using the Cell Counting Kit-8 (CCK-8) assay after a 48-hour incubation period.
Results and Key Data: - The combination treatment exhibited a significantly lower half-maximal inhibitory concentration (IC50) of 5.44 ng/ml compared to CTX alone (17.74 ng/ml), indicating a greater efficacy of the combination therapy.
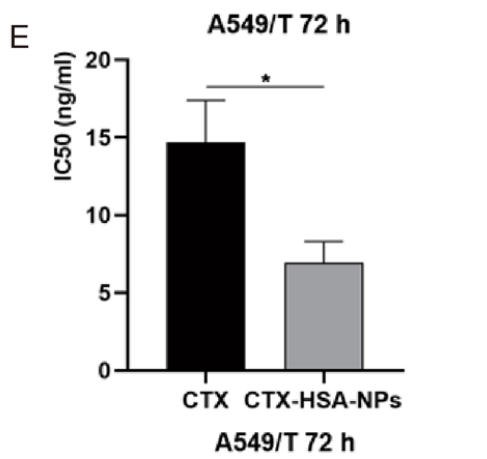
Figure 2. IC50 of cabazitaxel API and CTX-HSA-NPs on A549/T cells
Significance of the Result:
- The enhanced cytotoxicity demonstrated by the combination therapy underscores its potential for effectively targeting drug-resistant cancer cells.
Key Innovations: - This study illustrates the synergistic effect of combining traditional chemotherapy with gene silencing, addressing multiple pathways of drug resistance simultaneously.
- In Vivo Efficacy Study
Key Steps:
- BALB/c nude mice bearing A549/T tumors were randomly divided into groups and treated with CTX-HSA-NPs, TGFβ-1 siRNA LNP, and their combination.
- Tumor growth was monitored, and tumor tissues were collected after 25 days for assessment of tumor inhibition rates (TIR) and immunohistochemical analysis.
Results and Key Data: - The combination therapy resulted in a TIR of 71.55%, significantly higher than the 50.52% observed with CTX-Tween.
Significance of the Result: - These results demonstrate the potential of the combination therapy to enhance antitumor efficacy in vivo, providing a promising strategy for managing drug-resistant NSCLC.
Key Innovations: - The successful application of dual therapy (chemotherapy plus siRNA) in a preclinical model showcases a novel approach to combatting multi-drug resistance in cancer treatment.
- Toxicity Assessment
Key Steps:
- Acute toxicity studies were performed on ICR mice treated with varying doses of CTX-HSA-NPs and CTX-Tween. – LD50 values were calculated based on observed mortality and adverse effects over a 28-day observation period.
Results and Key Data: - The LD50 for CTX-HSA-NPs was found to be 190.609 mg/kg, indicating a significantly higher tolerance compared to 67.648 mg/kg for CTX-Tween, with a toxicity reduction of 1.8 times.
Significance of the Result: - The reduced toxicity profile of CTX-HSA-NPs suggests a safer alternative for patients, allowing for potentially higher doses and improved therapeutic outcomes.
Key Innovations: - The use of human serum albumin as a carrier significantly mitigates the toxic effects typically associated with chemotherapy, enhancing patient safety while maintaining efficacy.
Conclusion
The study concludes that CTX-HSA-NPs effectively reduce the toxicity associated with traditional CTX formulations while demonstrating superior anti-tumor activity against paclitaxel-resistant NSCLC. The incorporation of TGFβ-1 siRNA LNP in the treatment regimen not only enhances efficacy but also provides a targeted approach to combat drug resistance.
Despite the promising results, the study acknowledges limitations, such as the need for further exploration into the mechanisms of action for the combination therapy. Future research should focus on clinical trials to validate these findings and further optimize the formulations.
This novel approach to treating drug-resistant NSCLC may pave the way for more effective cancer therapies, addressing one of the critical challenges in oncology today.
Reference
Tan, Tiantian, et al. “Cabazitaxel-loaded human serum albumin nanoparticles combined with TGFβ-1 siRNA lipid nanoparticles for the treatment of paclitaxel-resistant non-small cell lung cancer.” Cancer Nanotechnology 14.1 (2023): 70.
