This study develops hyaluronic acid-modified PLGA magnetic nanoparticles for dual-targeted cisplatin delivery, combining magnetic guidance and NIR-responsive photothermal therapy to enhance the efficacy and reduce side effects in cancer treatment.
Key Preview
Research Question
This study investigates the effectiveness of hyaluronic acid (HA)-modified poly(lactic-co-glycolic acid) (PLGA) magnetic nanoparticles (MNPs) in enhancing cisplatin (CDDP) delivery for dual-targeted near-infrared (NIR)-responsive chemo-photothermal cancer therapy.
Research Design and Strategy
A combination of in vitro and in vivo experiments was used, including nanoparticle characterization, cytotoxicity testing on U87 glioblastoma cells, and tumor growth assessments in nude mice.
Method
HA-modified cisplatin-encapsulated PLGA MNPs were synthesized using a single emulsification/solvent evaporation method, followed by surface modification with HA, and tested for cytotoxicity and apoptosis in vitro, alongside in vivo tumor growth inhibition.
Key Results
The HA/PMNPc nanoparticles showed significantly enhanced cytotoxicity (IC50 = 0.297 µg/mL) compared to free CDDP (IC50 = 0.648 µg/mL), and in vivo studies demonstrated prolonged survival and inhibited tumor growth in mice.
Significance of the Research
This research demonstrates the potential of HA-modified PLGA MNPs as a targeted cisplatin delivery system, combining magnetic targeting and NIR-responsive photothermal therapy to improve cancer treatment efficacy while minimizing side effects.
Introduction
The treatment landscape for cancer has evolved significantly over the decades, with a focus on reducing the side effects of chemotherapeutics while enhancing their potency. Cisplatin, a widely used chemotherapeutic agent, has shown efficacy against several cancer types, but its clinical use is limited by severe side effects and the development of drug resistance. Recent advancements in nanomedicine have proposed using nanoparticles to improve drug delivery systems, particularly for cancer therapy. This study introduces a novel approach by utilizing HA-modified PLGA magnetic nanoparticles for dual-targeted delivery of cisplatin, aiming to enhance therapeutic efficacy while minimizing adverse effects.
Research Team and Objective
The research was conducted by a team of experts at Chang Gung University, including Huai-An Chen, Yu-Jen Lu, Banendu Sunder Dash, Yin-Kai Chao, and Jyh-Ping Chen. The study, titled “Hyaluronic Acid-Modified Cisplatin-Encapsulated Poly(Lactic-co-Glycolic Acid) Magnetic Nanoparticles for Dual-Targeted NIR-Responsive Chemo-Photothermal Combination Cancer Therapy,” was published in the journal Pharmaceutics. The objective was to develop a dual-targeted drug delivery system that could effectively deliver cisplatin to cancer cells while reducing systemic toxicity.
Experimental Process
Outline:
- Synthesis of Iron Oxide Magnetic Nanoparticles (IOMNPs)
- Preparation of Cisplatin-Loaded PLGA Magnetic Nanoparticles (PMNPc)
- Surface Modification with Chitosan and Hyaluronic Acid
- In Vitro Characterization of Nanoparticles
- In Vivo Study
1. Synthesis of Iron Oxide Magnetic Nanoparticles (IOMNPs)
Key Steps:
The first step involved the synthesis of iron oxide magnetic nanoparticles (IOMNPs) using the co-precipitation method. In a three-necked flask, 2.15 g of iron chloride hexahydrate (FeCl₃·6H₂O) and 0.79 g of iron chloride tetrahydrate (FeCl₂·4H₂O) were dissolved in 50 mL of deionized water. After purging the solution with nitrogen gas for 10 minutes, 5 mL of ammonium hydroxide (NH₄OH) was added dropwise to induce the precipitation of iron oxide nanoparticles. The reaction was kept at 60°C under continuous stirring for 30 minutes. The nanoparticles were then washed with deionized water using magnetic separation.
Results and Key Data:
The resulting iron oxide nanoparticles displayed a mean size of approximately 10–20 nm, as confirmed by transmission electron microscopy (TEM). The X-ray diffraction (XRD) analysis revealed six characteristic peaks corresponding to the magnetite structure, ensuring the formation of iron oxide particles.
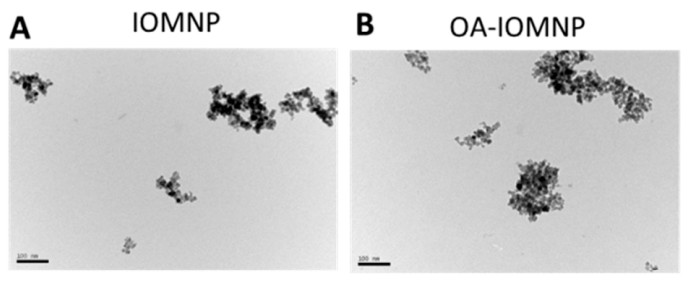
Figure 1. The transmission electron microscope (TEM) images of iron oxide magnetic nanoparticles (IOMNP) ((A) bar = 100 nm), oleic acid (OA)-coated IOMNP (OA-IOMNP) ((B) bar = 100 nm), CDDP-loaded PLGA magnetic nanoparticles (PMNPc).
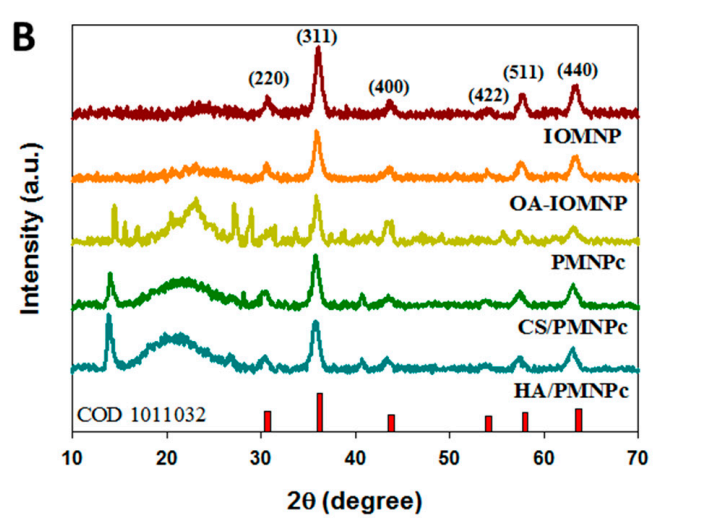
Figure 2. X-ray diffraction (XRD) analysis
Significance of the Results:
The successful synthesis of IOMNPs is a critical foundation for the subsequent steps in nanoparticle preparation. These particles exhibit superparamagnetic properties, making them suitable for both magnetic targeting and NIR-induced photothermal therapy. The size of the particles is optimal for drug encapsulation and intracellular uptake.
Key Innovations:
The use of a co-precipitation method for synthesizing the magnetic nanoparticles ensured a high degree of control over the particle size and uniformity, addressing common issues of aggregation in conventional methods.
2. Preparation of Cisplatin-Loaded PLGA Magnetic Nanoparticles (PMNPc)
Key Steps:
Next, the poly(lactic-co-glycolic acid) (PLGA) magnetic nanoparticles (PMNPc) were prepared by co-encapsulating cisplatin (CDDP) and the oleic acid-coated IOMNPs (OA-IOMNP). To prepare the PLGA matrix, 50 mg of PLGA was dissolved in a solvent mixture of acetone and dichloromethane (DCM). To this solution, 0.5 mL of a 10 mg/mL OA-IOMNP suspension was added. The mixture was sonicated for 1 minute and then added to a beaker containing 24 mL phosphate-buffered saline (PBS, pH 7.4) and 0.3% polyvinyl alcohol (PVA). The emulsion was then stirred at 35°C for 12 hours to allow solvent evaporation, resulting in the formation of nanoparticles encapsulating both CDDP and IOMNPs.
Results and Key Data:
The resulting PMNPc particles had an average size of 242.2 nm, as measured by dynamic light scattering (DLS). The encapsulation efficiency (EE) of CDDP in the nanoparticles was found to be 62.1%, and the loading capacity (LC) was approximately 18%. This demonstrates effective encapsulation of the chemotherapeutic agent.
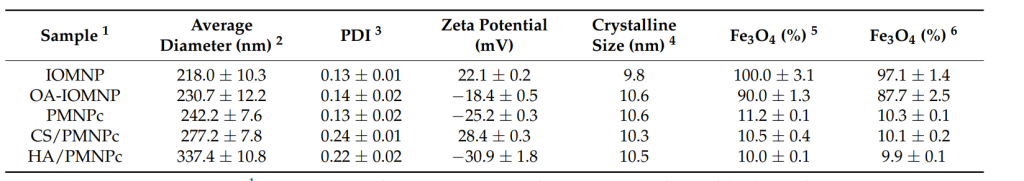
Table 1. The properties of different nanoparticles.
Significance of the Results:
The encapsulation of both CDDP and IOMNPs in PLGA nanoparticles represents a dual-function platform for chemo-photothermal therapy. The high encapsulation efficiency of CDDP ensures that a therapeutic dose is delivered to the target site, while the magnetic properties of the nanoparticles enable targeted drug delivery.
Key Innovations:
The co-encapsulation of both a chemotherapeutic agent and magnetic nanoparticles in a PLGA matrix is a novel approach that addresses the dual therapeutic needs of targeted drug delivery and photothermal therapy. This method enhances drug stability and improves targeted delivery, overcoming challenges faced by traditional drug delivery systems.
3. Surface Modification with Chitosan and Hyaluronic Acid
Key Steps:
The PMNPc were then sequentially surface-modified with chitosan (CS) and hyaluronic acid (HA) to enhance their targeting ability and biocompatibility. To begin, the PMNPc suspension (1 mg/mL) was added dropwise into a 50 mL chitosan solution (2% w/v) prepared in 1% acetic acid. The mixture was stirred for 1 hour at room temperature to obtain CS-coated nanoparticles (CS/PMNPc). Following this, the CS/PMNPc particles were added to a 50 mL HA solution (2% w/v) and stirred for another hour to produce the final HA-modified nanoparticles (HA/PMNPc).
Results and Key Data:
The surface modification significantly increased the particle size of the nanoparticles from 242.2 nm (PMNPc) to 337.4 nm (HA/PMNPc). Zeta potential measurements showed that the zeta potential changed from −25.2 mV (PMNPc) to −30.9 mV (HA/PMNPc), confirming successful modification with HA, a negatively charged polysaccharide.
Significance of the Results:
The modification of the nanoparticles with HA enhances their targeting ability towards CD44 overexpressing cancer cells, such as U87 glioblastoma cells. HA’s biocompatibility also reduces the likelihood of immune response, allowing for better circulation times and more efficient tumor targeting.
Key Innovations:
The dual-layer modification with chitosan and HA is an innovative approach that combines the advantages of both polymers—chitosan for stability and HA for targeting specificity. This method provides a significant improvement over previous nanoparticle modifications that used only one surface-modifying agent.
4. In Vitro Characterization of Nanoparticles
Key Steps:
To evaluate the nanoparticles’ size distribution, zeta potential, and morphology, the HA/PMNPc were analyzed using dynamic light scattering (DLS), transmission electron microscopy (TEM), and Fourier-transform infrared spectroscopy (FTIR). Cytotoxicity of the nanoparticles was assessed by incubating U87 glioblastoma cells with varying concentrations of HA/PMNPc, and cell viability was measured using the MTT assay.
Results and Key Data:
TEM analysis revealed that the nanoparticles retained a spherical morphology, with sizes around 337.4 nm. DLS confirmed a uniform size distribution with a polydispersity index (PDI) below 0.3, indicating good colloidal stability. The cytotoxicity study showed that HA/PMNPc exhibited an IC50 of 0.297 µg/mL, which was significantly lower than that of free CDDP (0.648 µg/mL), indicating enhanced drug delivery efficiency.
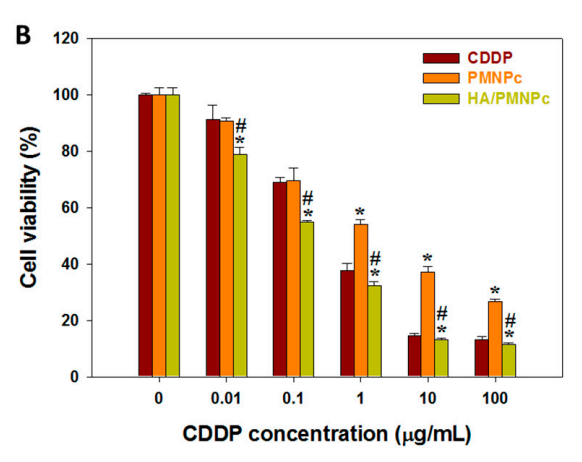
Figure 3. (B) The cytotoxicity of CDDP, CDDP-loaded PLGA magnetic nanoparticles (PMNPc), and HA-modified PMNPc (HA/PMNPc) after incubating with U87 cells for 72 h and determining the cell viability by MTT assays. * p < 0.05 compared with CDDP, # p < 0.05 compared with PMNPc.
Significance of the Results:
The in vitro results confirmed that the HA-modified nanoparticles had improved cytotoxicity against U87 cells, demonstrating the effectiveness of HA for active targeting. The uniform size and high colloidal stability of the nanoparticles suggest that they will maintain their therapeutic efficacy in vivo.
Key Innovations:
The use of a combination of DLS, TEM, and FTIR for the characterization of nanoparticles allowed for a comprehensive understanding of their size, morphology, and composition. This multi-faceted approach ensures that the nanoparticles meet the necessary criteria for drug delivery and targeting efficacy.
5. In Vivo Study
Key Steps:
The in vivo anticancer efficacy was evaluated in nude mice implanted with U87 glioblastoma cells. The HA/PMNPc were administered intravenously, and magnetic targeting was applied using a 2400 Gauss magnet. NIR laser irradiation was applied to the tumor area to trigger the photothermal effect. Tumor volume and survival times were monitored, and histological analysis was performed on tumor tissues.
Results and Key Data:
In vivo results demonstrated that the HA/PMNPc with magnetic targeting and NIR laser treatment (HA/PMNPc (M+/L+)) resulted in the lowest tumor growth and the longest median survival time (42 days) compared to other treatment groups. Bioluminescence imaging (BLI) and histological analysis confirmed efficient tumor inhibition and increased apoptosis in the tumor tissue.
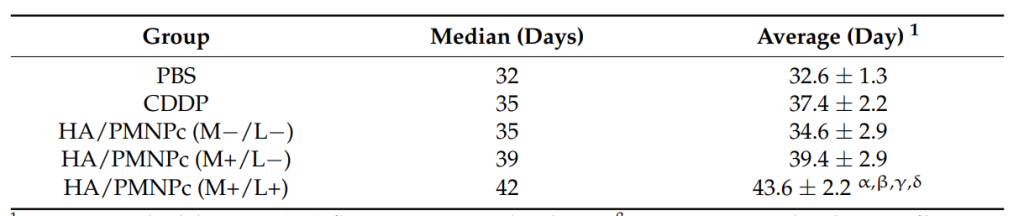
Table 2. Mean ± standard deviation (SD). α p < 0.05 compared with PBS, β p < 0.05 compared with CDDP, γ p < 0.05 compared with HA/PMNPc (M−/L−), δ p < 0.05 compared with HA/PMNPc (M+/L-).
Significance of the Results:
These in vivo findings strongly support the dual-targeted approach, highlighting the combined benefit of magnetic targeting and NIR-responsive photothermal therapy for improved therapeutic outcomes. The extended survival times of the treated mice underline the potential of HA/PMNPc in clinical cancer therapy.
Key Innovations:
The combination of magnetic targeting and photothermal therapy in the in vivo experiments offers a novel and effective strategy for cancer treatment, improving drug delivery and therapy precision. The use of a non-invasive magnetic guidance system coupled with NIR irradiation represents a significant advancement over traditional treatments.
Conclusion
The study successfully demonstrated that HA-modified PLGA MNPs can enhance the delivery of cisplatin for targeted cancer therapy. Key findings indicated a significant reduction in tumor growth and improved survival rates in treated mice. While the research established a promising foundation for utilizing dual-targeted therapies, it also acknowledged limitations, such as the need for further studies to explore long-term effects and potential resistance mechanisms. Future research directions may involve refining the nanoparticle formulations and exploring other combinations of therapies to enhance cancer treatment efficacy.
Reference:
Chen, Huai-An, et al. “Hyaluronic acid-modified cisplatin-encapsulated poly (lactic-co-glycolic acid) magnetic nanoparticles for dual-targeted NIR-responsive chemo-photothermal combination cancer therapy.” Pharmaceutics 15.1 (2023): 290.
