Editor: Nina
This study presents the development of an injectable, photoactive hydrogel incorporating BiH nanorods and allantoin for combined cancer photothermal therapy, wound healing, antibacterial, and hemostatic functions, demonstrating its potential for improved therapeutic outcomes.
Key Preview
Research Question: The key question driving this research is how to effectively integrate cancer thermotherapy with wound healing and antimicrobial functions into a single injectable hydrogel formulation to address the complications of traditional cancer treatments.
Research Design and Strategy: The study uses a systematic approach to create a multifunctional hydrogel by combining photothermal agents and regenerative drugs, utilizing metal-coordination chemistry to deliver localized photothermal therapy, promote wound healing, and prevent infections.
Method: The hydrogel is developed using Bi2S3 nanorods as photothermal agents, coated with hyaluronic acid for stability, and combined with allantoin in a sodium alginate and Farsi gum-based matrix, with Fe3+-mediated coordination for effective delivery and performance evaluation through in vitro and in vivo experiments.
Key Results: The hydrogel demonstrated excellent photothermal conversion efficiency, effectively inducing tumor cell necrosis while promoting tissue repair, accelerating wound healing, eradicating bacteria, and exhibiting strong blood-clotting capabilities.
Significance of the Research: This study introduces a novel multifunctional hydrogel that not only targets tumors but also enhances recovery and minimizes infection risk, offering a promising solution for improving patient outcomes in cancer therapy.
Introduction
The evolution of cancer treatment has seen substantial advancements over the years, yet traditional therapies like surgery, chemotherapy, and radiotherapy often fail to provide optimal results. Current methods do not sufficiently eradicate tumor cells, leading to a substantial percentage of cancer-related deaths. Moreover, the post-surgical recovery phase is frequently marred by inflammation and potential infections, which can significantly delay wound healing. The development of innovative therapeutic approaches remains imperative, particularly those capable of addressing the dual challenges of cancer treatment and wound management.
Recent breakthroughs in nanotechnology and biomaterials have opened new avenues for treatment strategies. The current study introduces a novel injectable hydrogel designed to provide simultaneous cancer therapy and wound healing. The hydrogel leverages metal-coordination synthesis to create a formulation that is not only effective in photothermal tumor ablation but also promotes rapid tissue regeneration. This multifaceted approach seeks to address the gaps in existing cancer therapies, emphasizing the need for integrated solutions that enhance patient recovery and survival.
Research Team and Objective
The research team comprises Kiyan Musaie, Samin Abbaszadeh, Vahideh Nosrati-Siahmazgi, Mostafa Qahremani, Shige Wang, Mohammad Reza Eskandari, Seyyed Vahid Niknezhad, Fakhri Haghi, Yulin Li, Bo Xiao, and Mohammad-Ali Shahbazi. Conducted at Zanjan University of Medical Sciences and published in Biomaterials Science, the study titled “Metal-coordination synthesis of a natural injectable photoactive hydrogel with antibacterial and blood-aggregating functions for cancer thermotherapy and mild-heating wound repair” aims to develop a multifunctional hydrogel that can effectively tackle the challenges faced in cancer treatment and wound healing.
Experimental Process
1. Synthesis of BiH Nanorods
Key Steps:
The Bi2S3 nanorods (BiH) were synthesized using a hydrothermal method, followed by coating with hyaluronic acid (HA). HA helps stabilize the BiH NRs and reduces aggregation, making them suitable for biological applications.
Synthesis Method: A solution of Bi2S3 was heated in the presence of HA to facilitate the formation of BiH nanorods. The process was controlled to ensure that the rods were formed with consistent size and shape.
Characterization: The morphology of the nanorods was confirmed using Transmission Electron Microscopy (TEM) and Field Emission Scanning Electron Microscopy (FE-SEM). Zeta potential measurements showed a change from -52.9 mV for Bi2S3 to -15.1 mV for BiH, confirming the successful coating of the nanorods.
Results and Key Data:
The BiH nanorods were found to be rod-shaped and exhibited good colloidal stability in deionized water, reducing aggregation over 24 hours.

Figure 1.Colloidal stability of prepared NRs after 24 h of dispersion in DW.
Significance of the Results:
The HA coating enhances the biocompatibility of BiH NRs, improving their stability in aqueous solutions, a key requirement for biomedical applications like photothermal therapy.
Key Innovations:
The HA-coating on BiH NRs not only improved their dispersion but also contributed to their colloidal stability, which is a unique aspect compared to non-coated Bi2S3 nanorods.
2. Hydrogel Preparation
Key Steps:
The hydrogels were prepared by mixing sodium alginate (Alg) and Farsi gum (FG), followed by the addition of ferric chloride hexahydrate (FeCl3·6H2O) as a crosslinking agent. BiH nanorods and allantoin (Alla) were incorporated into the hydrogel mixture.
Gelation: The FeCl3·6H2O facilitated the crosslinking between the hydroxyl and carboxyl groups of Alg and FG, forming a metal-coordinated bond.
Characterization: The gelation time, porosity, water content, and yield were evaluated, revealing high porosity (>80%) and water content (79.2 ± 1.7%) for the Gel and Gel-BiH formulations.
Results and Key Data:
The gelation occurred almost instantly upon adding FeCl3·6H2O, taking approximately 5 seconds.
The incorporation of BiH did not significantly affect the gelation process, porosity, or water retention capacity.
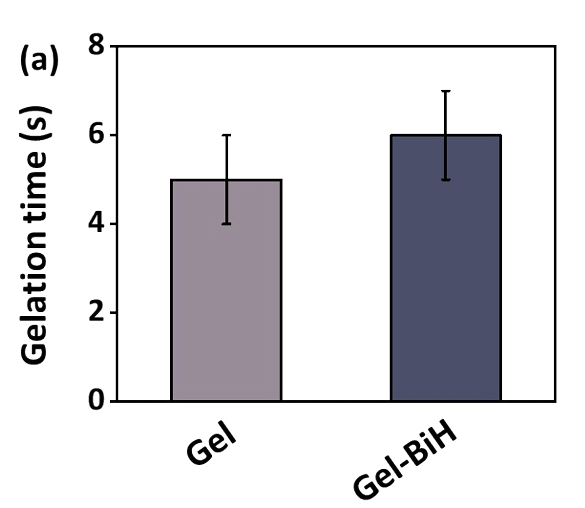
Figure 2.(a) The gelation time of Gel and Gel-BiH hydrogels.
Significance of the Results:
The high porosity ensures efficient oxygen and nutrient transfer in wound healing, while the high water content allows for better absorption of exudates from wounds.
Key Innovations:
The use of a metal-coordinated bond between Alg, FG, and Fe3+ to form a hydrogel with self-healing properties is a novel design, allowing the hydrogel to remain stable under physiological conditions while enabling easy injection.
3. Photothermal Therapy (PTT) Evaluation
Key Steps:
The photothermal properties of the Gel-BiH hydrogel were evaluated under 808 nm near-infrared (NIR) laser irradiation. The hydrogels were exposed to varying laser power densities (0.65 W cm−2, 1 W cm−2, and 1.5 W cm−2) for 10 minutes to assess temperature changes.
Temperature Measurement: The temperature increase in the hydrogel was recorded using an infrared thermographic camera.
Results and Key Data:
The temperature of the Gel–BiH hydrogel increased to 45.4°C at 1 W cm−2 and 50.3°C at 1.5 W cm−2, demonstrating efficient photothermal conversion.
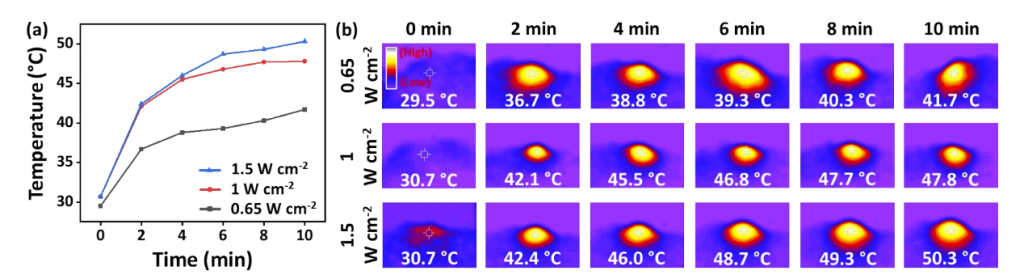
Figure 3.(a) The temperature elevation curve of Gel-BiH injected under the skin of BALB/c mice and followed by the irradiation of the 808 nm laser for 10 min (0.65, 1, and 1.5 W cm-2) and (b) representative infrared thermal images.
Significance of the Results:
The ability of the hydrogel to reach temperatures exceeding 40°C is essential for photothermal cancer therapy, where temperatures around 50°C can induce tumor cell necrosis. The temperature control in the Gel–BiH formulation supports both cancer treatment and mild heating for wound healing.
Key Innovations:
The Gel–BiH hydrogel’s capability to undergo repeated cycles of NIR irradiation while maintaining temperature stability allows for multiple rounds of therapy without degradation, enhancing its therapeutic potential.
4. In Vivo Tumor Treatment and Wound Healing Studies
Key Steps:
For in vivo tumor therapy, the Gel–BiH hydrogel was injected into tumor-bearing mice followed by NIR irradiation to evaluate its effectiveness in tumor ablation. Simultaneously, wound healing studies were conducted by creating skin defects in animals treated with various formulations.
Results and Key Data:
The Gel–BiH hydrogel demonstrated significant tumor volume reduction after treatment with NIR irradiation. Wound healing was faster in the Gel–BiH and Gel–BiH–Alla groups compared to control groups, with a 12% higher closure rate on day 3 and reduced wound area at day 14.
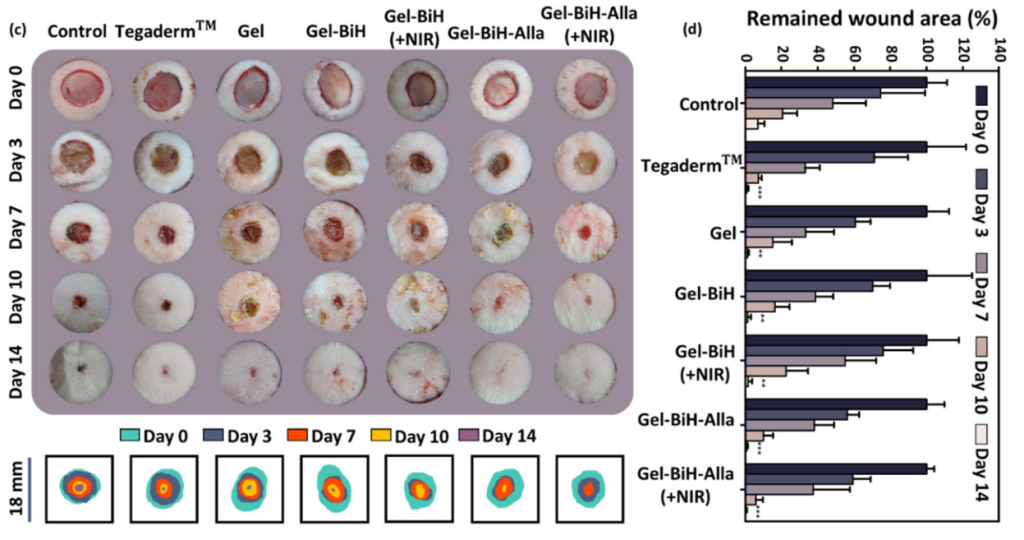
Figure 4. (c) Skin photographs of wound closure in full-thickness wound model on days 0, 3, 7, 10, and 14 in the control group, Tegaderm™, Gel, Gel–BiH ± NIR, and Gel–BiH–Alla ± NIR treated groups (808 nm, 1 W cm−2 , 3 min). Photographs were taken from a representative animal of each group. (Scale bar = 18 mm.) (d) The percentage of the remaining wound area for referred groups over 14 days of screening. Data are presented as the mean ± SD (N = 5). Statistical analysis was separately conducted for all groups compared to the control group using one-way ANOVA on the same day (**p < 0.01, ***p < 0.001).
Significance of the Results:
The dual-functionality of the hydrogel—cancer ablation and wound healing—illustrates its potential for combined cancer therapy and tissue regeneration in a single treatment.
Key Innovations:
The unique combination of photothermal therapy with allantoin-mediated tissue regeneration in the same hydrogel formulation offers a powerful, multifunctional treatment platform that addresses both tumor suppression and accelerated wound healing simultaneously.
Conclusion
In summary, the study presents an injectable hydrogel that integrates photothermal therapy, antibacterial properties, and regenerative capabilities, offering a promising approach to combat cancer and enhance wound healing. The hydrogel demonstrated effective tumor ablation and expedited healing processes, primarily due to its multifunctional properties. While the findings are promising, further research is necessary to explore its long-term efficacy and safety in clinical settings. Future studies should aim to optimize the formulation and evaluate its performance across different types of cancers and wound conditions, ultimately contributing to improved therapeutic options for patients facing these challenges.
Reference:
Musaie, Kiyan, et al. “Metal-coordination Synthesis of a Natural Injectable Photoactive Hydrogel with Antibacterial and Blood-aggregating Functions for Cancer Thermotherapy and Mild-heating Wound Repair.” Biomaterials Science, vol. 11, no. 11, 2023, pp. 2486–2503.
