Author: Tiffany
A novel strategy utilizing a sonodynamic-immunomodulatory nanostimulator has demonstrated the potential to activate pyroptosis and modify the tumor microenvironment, thus offering a promising approach for improving cancer immunotherapy outcomes.
Exploring Pyroptosis in Cancer Treatment
Introduction to Pyroptosis
Pyroptosis is a type of programmed cell death marked by cell swelling, membrane disruption, and the release of pro-inflammatory molecules. This form of cell death plays a significant role in cancer immunotherapy due to its ability to initiate and amplify an immune response. In contrast to other types of cell death, such as apoptosis, pyroptosis facilitates a more robust activation of the immune system, which could potentially improve the effectiveness of cancer treatments.
Challenges in Current Cancer Therapies
Despite advances in cancer treatment, many conventional therapies continue to face significant limitations, such as tumor resistance to drugs and ineffective targeting of deep tissue tumors. A major obstacle in treating solid tumors is the dense extracellular matrix (ECM), which serves as a physical barrier that prevents the effective infiltration of immune cells. These barriers complicate the delivery and efficacy of treatments, highlighting the need for more advanced strategies to enhance immune response and improve therapeutic outcomes.
Potential of Sonodynamic Therapy (SDT)
Sonodynamic therapy (SDT) has emerged as a promising therapeutic option that uses ultrasound to activate sonosensitizers, generating reactive oxygen species (ROS) that can cause tumor cell death. Compared to photodynamic therapy (PDT), SDT offers several advantages, including deeper tissue penetration and a reduced risk of side effects. This makes it a particularly viable approach for inducing pyroptosis in deep-seated tumors, potentially overcoming some of the limitations faced by traditional therapies.
Research Objective and Goals
The main aim of this study was to develop an approach that combines sonodynamic therapy with an immunomodulatory strategy to activate pyroptosis and remodel the tumor microenvironment. By targeting both tumor cells and the surrounding immune environment, this combined strategy aims to improve the efficacy of cancer immunotherapy, particularly by increasing T-cell infiltration and reducing ECM barriers.
This research was carried out by a team from the Lab of Molecular Imaging and Translational Medicine at Xidian University. The results were published in Theranostics in 2023, presenting a new approach to tumor immunotherapy that integrates multiple therapeutic strategies to address current limitations.
Research Methods and Results
Experimental Process Overview:
- Synthesize LY364947-loaded PCN-224 nanoparticles.
- Coat PCN-224 nanoparticles with red blood cell (RBC) membranes to form LPM.
- Assess the size and zeta potential of LPM to confirm successful preparation.
- In vitro testing:
Expose 4T1 cells to LPM and ultrasound (US) for ROS generation.
Evaluate pyroptosis in 4T1 cells through cell morphology, MTT assay, Annexin V/PI staining, and Western blot for GSDME activation.
Measure cell death and cytokine release (IL-1β, IL-18). - In vivo testing:
Administer LPM + US to 4T1 tumor-bearing mice for tumor treatment.
Assess ECM collagen depletion via immunofluorescence.
Measure T-lymphocyte infiltration using CD3+ and CD8+ T-cell markers.
Evaluate tumor volume and weight to determine therapeutic effect. - Analyze immune response and tumor memory:
Conduct tumor re-challenge experiments to assess immune memory.
Measure the proliferation of memory T cells (CD62L+CD44+ and CD62L−CD44+) in spleen cells post re-challenge.
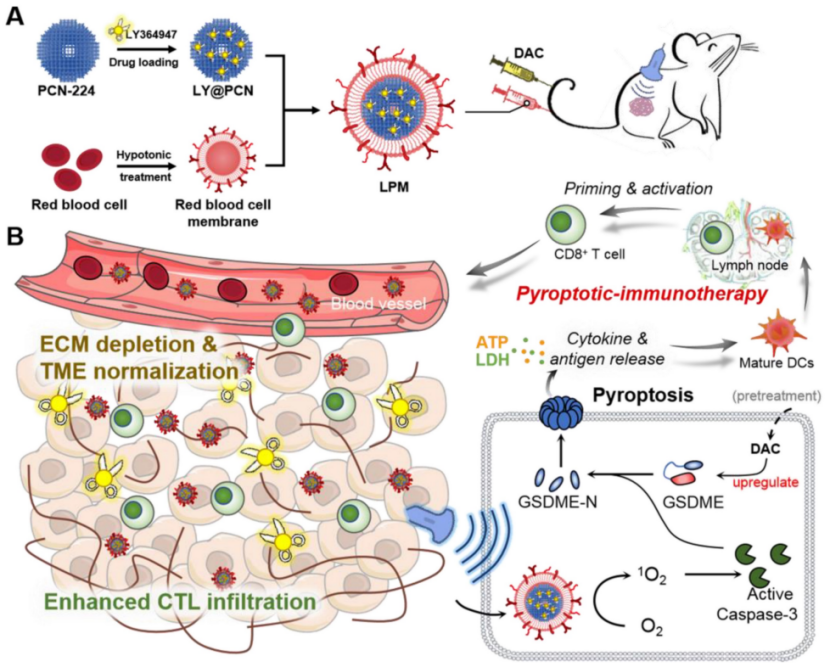
Figure 1. Schematic illustration of anti-tumor immunotherapy induced by the sonodynamic-immunomodulatory nanostimulator LPM.
Key Experiments and Detailed Results:
- Preparation of the Nanostimulator and Its Properties:
Preparation: LY364947 was loaded into PCN-224 nanoparticles, which were then camouflaged with RBC membranes to enhance circulation and tumor targeting.
Results: The resulting nanostimulator (LPM) exhibited a hydrodynamic diameter of 116.0 ± 2.3 nm, which was larger than bare PCN-224 nanoparticles (90 nm). The zeta potential shifted from 8.7 ± 0.5 mV to −18.9 ± 0.9 mV after RBC membrane coating, indicating successful encapsulation and membrane coating.
New Findings: The RBC membrane coating did not affect the sonosensitivity of the nanostimulator, as shown by consistent ROS generation upon ultrasound irradiation.
- Induction of Pyroptosis via SDT and GSDME Activation:
Preparation: 4T1 cells were pretreated with DAC to upregulate GSDME expression, and LPM was applied to these cells.
Procedure: Cells were exposed to ultrasound (US) irradiation (0.5 W cm−2, 50% duty ratio, 1 MHz for 5 minutes). The activation of pyroptosis was confirmed by observing typical pyroptotic morphology under a microscope (swelling and bubble-like changes in cell shape).
Results: Cells treated with LPM and US showed a 76% reduction in cell viability, indicating efficient cell death. Flow cytometry analysis and Annexin V/PI staining confirmed the pyroptotic and apoptotic processes.
New Findings: Western blotting revealed a significant increase in GSDME-N levels in the DAC + LPM + US group, indicating that pyroptosis was successfully triggered via the caspase-3/GSDME pathway.
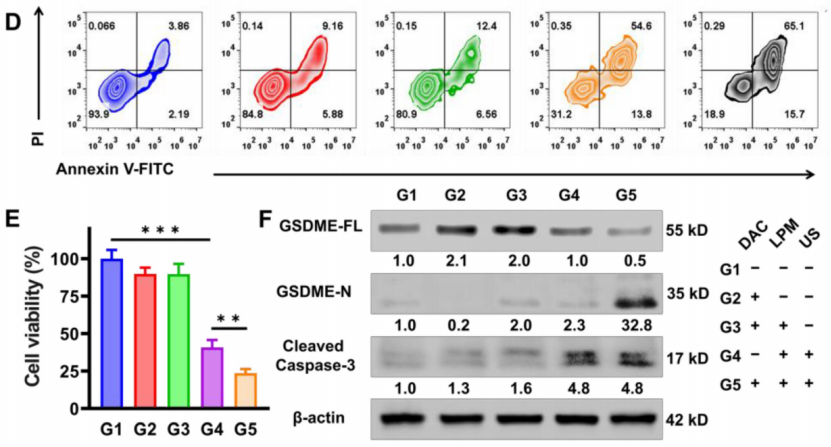
Figure 2. Pyroptosis triggering in vitro by SDT. (D) Flow cytometry analysis of Annexin V-FITC and PI co-stained 4T1 cells after different treatments. (E) The cytotoxicity assessment on 4T1 cells after different treatments. (F) Western blot analysis of GSDME-FL, GSDME-N and cleaved caspase-3 expression in 4T1 cells after different treatments.
- Tumor ECM Normalization and Immune Response Enhancement:
Preparation: LPM was administered to 4T1 tumor-bearing mice to test its effect on ECM normalization and immune infiltration.
Procedure: Tumors were analyzed for collagen depletion using immunofluorescence staining, and T-cell infiltration was assessed by counting CD3+ T cells in the tumor tissue.
Results: Collagen levels were significantly reduced in the LPM-treated group compared to the control, with a 48% depletion observed. T-lymphocyte infiltration increased by 1.9-fold, promoting a more uniform distribution of immune cells within the tumor.
New Findings: The ECM normalization allowed for deeper T-cell infiltration and enhanced immune responses, which are critical for amplifying the effects of pyroptosis-induced tumor immunotherapy.
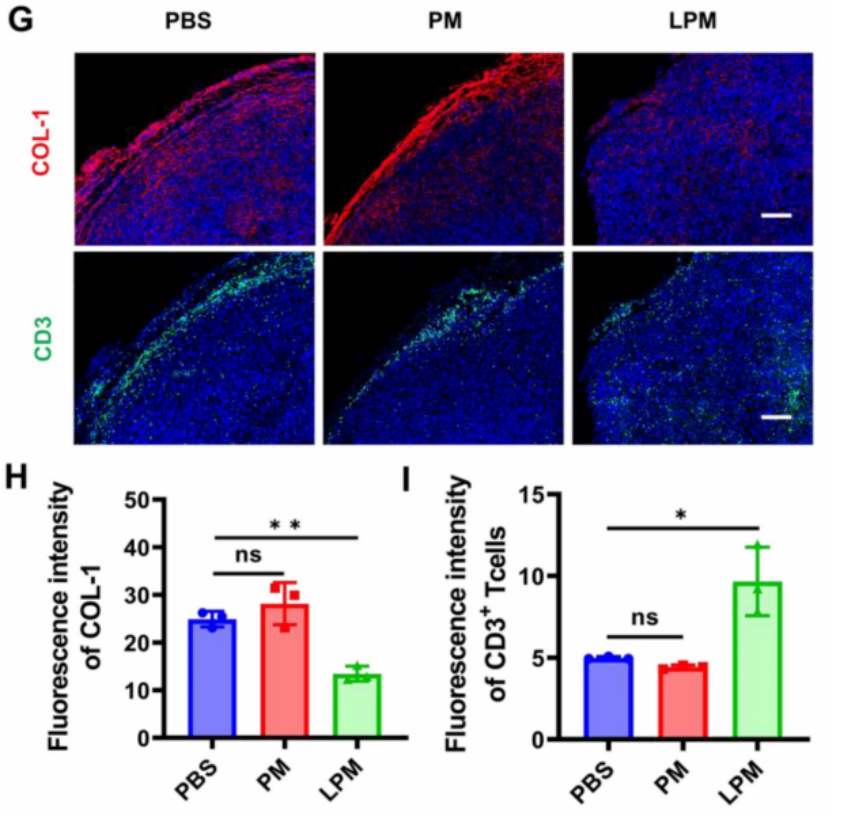
Figure 3. Normalization of tumor physical scaffold by collagen removing. (G) and quantification of collagen I (H) and CD3+T cells (I) in 4T1 tumors. Blue, cell nuclei staining; red, collagen I staining; green, CD3+T cells staining.
- In Vivo Tumor Eradication and Immune Memory Formation:
Preparation: 4T1 tumor-bearing mice were treated with LPM + US for 3 days, and tumor growth was monitored over 14 days.
Procedure: Tumor volumes were measured, and mice were sacrificed to evaluate tumor eradication, immune cell infiltration, and immunological memory.
Results: LPM + US treatment led to nearly complete tumor elimination, with 80% of the mice remaining tumor-free at the end of the study (Figure 4D). Immunohistochemical analysis showed a significant increase in CD8+ T-cell infiltration and dendritic cell maturation in the LPM + US group.
New Findings: The treatment induced strong immune memory, as evidenced by the ability of the LPM-treated mice to resist tumor re-challenge after 60 days (Figure 5B). Flow cytometry analysis revealed a 5.4-fold increase in central memory T cells (CD62L+CD44+) and a 6.4-fold increase in effector memory T cells (CD62L−CD44+), indicating a robust adaptive immune response.
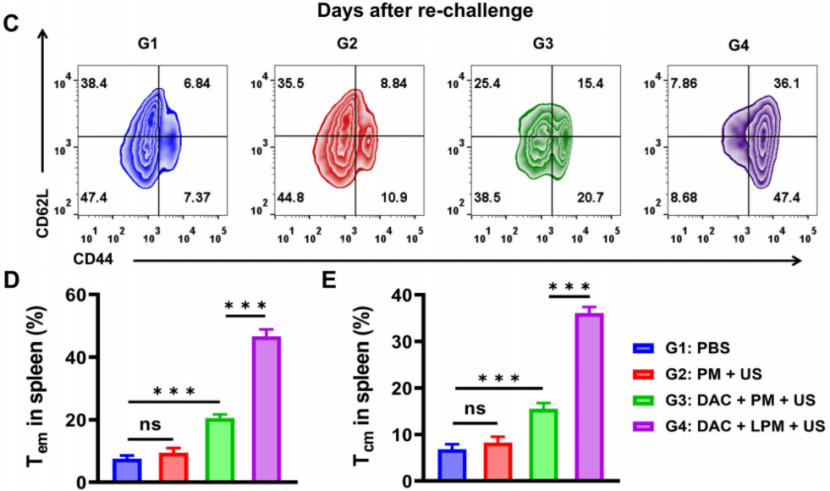
Figure 4. Long-term anti-tumor memory immune response. (C) Representative flow cytometric analysis of memory T cells within the spleen after tumor re-challenge assay. Absolute quantification of CD44+CD62L− cells gating on CD3+CD4+ cells (D) and CD44+CD62L+ cells gating on CD3+CD4+ cells (E). Data are presented as the mean ± SD.
IV. Study Summary and Key Findings
Study Findings: This research demonstrated the successful application of a sonodynamic-immunomodulatory nanostimulator that activates pyroptosis, enhances immune cell infiltration, and remodels the tumor microenvironment. The approach significantly improved tumor immunotherapy outcomes by increasing the infiltration of immune cells into tumors and reducing physical barriers that hindered immune response.
Innovation and Significance: This study highlights a novel approach to cancer immunotherapy that combines SDT, pyroptosis, and ECM remodeling. By targeting both the tumor cells and the tumor microenvironment, this method shows promise in overcoming several obstacles that limit the efficacy of traditional cancer treatments. The results offer valuable insights into the potential of using nanotechnology and sonodynamic therapy to improve cancer immunotherapy and establish long-lasting anti-tumor immunity.
Reference:
Chen, Z., W. Liu, and Z. Yang. “Sonodynamic-immunomodulatory nanostimulators activate pyroptosis and remodel tumor microenvironment for enhanced tumor immunotherapy. Theranostics. 2023; 13 (5): 1571–83.”
