Editor: Nina
Researchers investigate the role of protein corona formation on the surface of 19F-labeled PLGA nanoparticles, demonstrating how surface modifications enhance cellular uptake and improve 19F-MRI sensitivity for non-invasive cell tracking applications.
Key Preview
Research Question:
The study examines the influence of the protein corona on the cellular uptake of fluorinated nanoparticles and their effectiveness for enhancing ^19F-MRI imaging sensitivity.
Research Design and Strategy:
The research uses an experimental approach where PLGA nanoparticles are functionalized, tested for protein corona formation in various biological environments, and their impact on cellular uptake and imaging efficacy is assessed.
Method:
The study synthesizes PLGA nanoparticles loaded with PERFECTA, a superfluorinated molecule, and stabilizes them with various biocompatible coatings. The nanoparticles are characterized for size, charge, protein corona formation, and cellular uptake via ^19F-MRI in microglial cells.
Key Results:
Results show that nanoparticles that easily form a protein corona exhibit significantly improved cellular internalization and ^19F-MRI sensitivity, with sodium cholate and chitosan coatings enhancing ^19F uptake more than polyvinyl alcohol.
Significance of the Research:
This research contributes to advancing non-invasive ^19F-MRI imaging techniques, potentially improving cell-based therapy monitoring by emphasizing the importance of protein corona in enhancing nanoparticle uptake for better imaging sensitivity.
Introduction
The evolution of cell therapy as a promising treatment approach for numerous diseases has underscored the need for effective imaging technologies. Non-invasive imaging tools capable of tracking cellular biodistribution and therapy outcomes are crucial for the clinical translation of these therapies. Magnetic resonance imaging (MRI) has emerged as a safe and effective method for longitudinal studies, providing comprehensive data that can be useful in clinical settings. However, conventional MRI contrast agents have limitations, leading researchers to explore alternative options such as fluorinated nanoparticles for improved imaging specificity.
Recent advancements in fluorinated nanoparticles, particularly those utilizing ^19F-MRI, have demonstrated the potential for non-invasive tracking of labeled cells. Unlike conventional agents, fluorinated nanoparticles can leverage the absence of endogenous fluorine in human tissues, allowing for a clearer signal that is directly proportional to the quantity of ^19F atoms present. Despite these advancements, the sensitivity of ^19F-MRI remains a challenge, particularly when tracking small populations of cells. Therefore, this study focuses on the critical role of nanoparticle surface modifications and the formation of a protein corona, which can significantly influence cellular uptake and imaging efficacy.
Research Team and Objective
The research team, led by Lodovico Gatti, Cristina Chirizzi, Giulia Rotta, and several collaborators, conducted this study at the Politecnico di Milano and IRCCS San Raffaele Scientific Institute. The paper titled “Pivotal role of the protein corona in the cell uptake of fluorinated nanoparticles with increased sensitivity for ^19F-MR imaging” was published in the journal Nanoscale Advances. The objective of this research was to optimize the design of fluorinated nanoparticles through surface modifications, enhancing their interaction with biological environments to improve cellular labeling efficiency and ^19F-MRI detection sensitivity.
Experimental Process
Outline:
- Synthesis and Functionalization of 19F–PLGA Nanoparticles (NPs)
- Protein Corona (PC) Formation and Characterization
- Cellular Uptake Assay
- Magnetic Resonance Imaging (MRI) and Signal Quantification
- Flow Cytometry for Cellular Uptake Analysis
1. Synthesis and Functionalization of 19F–PLGA Nanoparticles (NPs)
Key Steps:
- Preparation of NPs: The nanoparticles were synthesized using poly(lactic-co-glycolic acid) (PLGA) as the base polymer, combined with the superfluorinated molecule PERFECTA for the fluorine label. PERFECTA was first dissolved in ethyl acetate (EtOAc), followed by PLGA dissolution in the same solvent. The solutions were then mixed, and the resulting organic solution was rapidly added dropwise to an aqueous stabilizer solution containing either sodium cholate (NaC), polyvinyl alcohol (PVA), or chitosan (CS).
- Stabilization and Coating: Depending on the stabilizer, the NPs were coated to have negative (NaC), neutral (PVA), or slightly positive (CS) electrokinetic potentials. The NP formulation was further processed by sonication to ensure uniform dispersion.
Results and Key Data:
- The resulting NPs were within the size range of 100–200 nm, with the specific size depending on the stabilizing agent used. The 19F–PLGA NPs showed high colloidal stability in aqueous solution, essential for maintaining particle integrity in biological applications.
- The surface charge of the NPs varied according to the stabilizer used: NaC-coated NPs exhibited a negative charge (-49 mV), PVA-coated NPs were neutral (-13 mV), and CS-coated NPs had a positive charge (~+19 mV).
- Encapsulation efficiency of PERFECTA, measured by 19F-NMR, ranged from 2–4 × 1020 atoms per mL, with NaC- and PVA–CS-coated NPs having the highest loading (~27 × 10^ 7 atoms per NP).
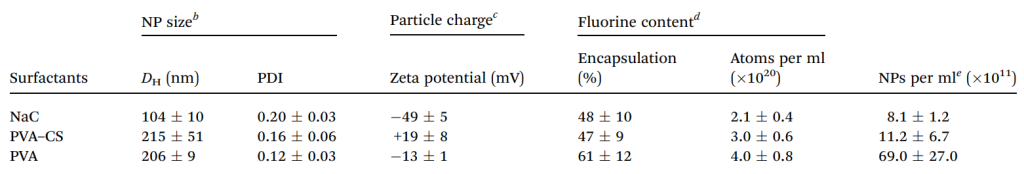
Table 1. Main physico-chemical features of PERFECTA PLGA probes a. All reported values are the mean of six independent measurements performed on different NP batches. b Hydrodynamic diameter (DH) and polydispersity index (PDI) obtained by cumulant fitting of DLS measurements. c Particle charge determined by z-potential measurement. d Fluorine content determined by 19 F-NMR. e NP concentration determined by Nanoparticle Tracking Analysis (NTA).
Significance of the Results:
- The controlled synthesis and functionalization of NPs allowed for the precise tuning of surface properties, which is crucial for optimizing cellular uptake. These surface modifications are key to the NPs’ interaction with biological systems, making them suitable for applications in cellular labeling and MRI.
Key Innovations:
- The design of NPs with varying electrokinetic potential and surface properties (negative, neutral, positive) enabled a systematic study of their interactions with biological media, especially the formation of the protein corona (PC).
- The development of a robust formulation process with high encapsulation efficiency for PERFECTA sets a new standard for preparing fluorine-labeled nanoparticles for MRI applications.
2. Protein Corona (PC) Formation and Characterization
Key Steps:
- Incubation with Serum Proteins: The NPs were incubated in DMEM culture medium supplemented with 10% fetal bovine serum (FBS) to simulate the biological environment. The NPs were exposed to serum proteins for various time points, from 5 minutes to 8 hours.
- Isolation of NP–Protein Complexes: After incubation, NP–protein complexes were isolated via sedimentation through a sucrose cushion. The NPs were washed and resuspended for further analysis.
- Characterization of the PC: The protein corona composition was analyzed using SDS-PAGE electrophoresis, which allowed for the identification of protein bands associated with the NPs. The NP size and aggregation were monitored by Dynamic Light Scattering (DLS).
Results and Key Data:
- NPs with NaC and CS coatings showed a significant increase in size (from 100 nm to over 200 nm) due to the formation of a protein corona, while PVA-coated NPs exhibited minimal changes in size.
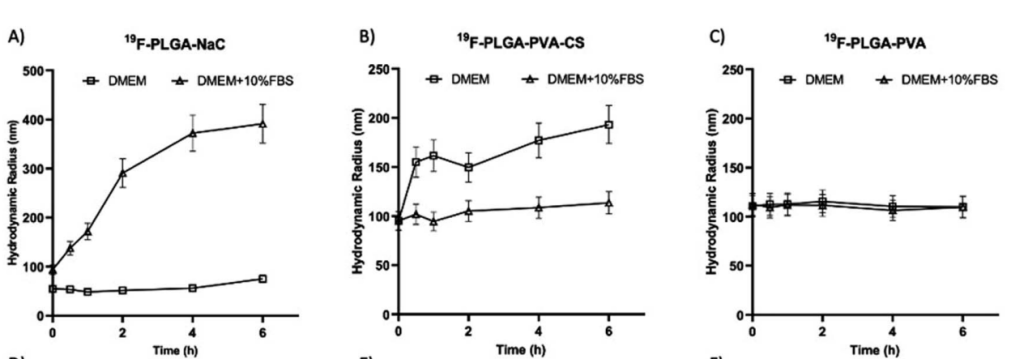
Figure 1. Hydrodynamic radius of all the NPs incubated in DMEM (square) or DMEM supplemented with 10% FBS (triangle) at 37 °C for time points from 5 min to 6 hours. Hydrodynamic radius s of PLGA–NaC (A), PLGA–PVA–CS (B) and PLGA–PVA (C).
- The SDS-PAGE analysis revealed a rich PC profile for NaC-coated NPs, with prominent protein bands at 75 and 25 kDa. In contrast, PVA-coated NPs did not form a significant protein corona, indicating less interaction with serum proteins.
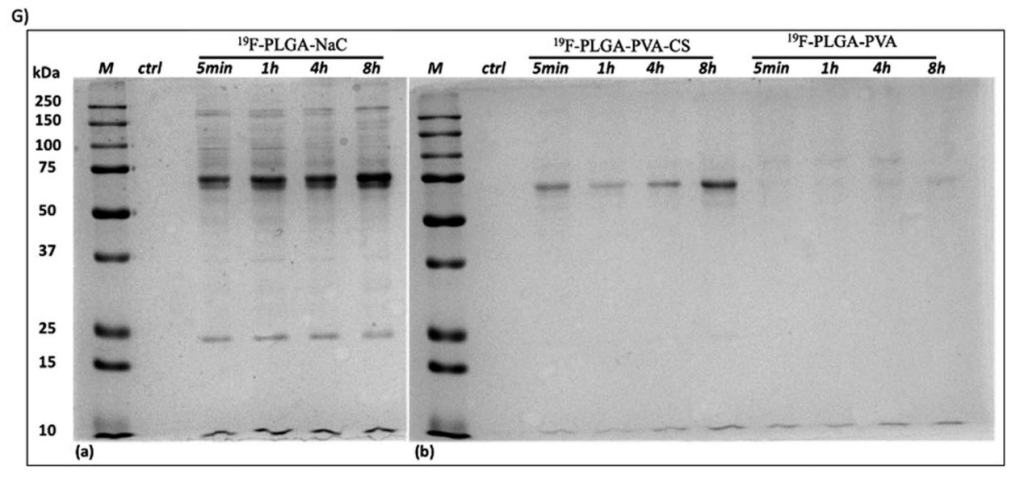
Figure 2. 1D-SDS– PAGE gel (G) of isolated HC PC from 19 F–PLGA–NaC NPs, 19 F–PLGA–PVA–CS and 19 F–PLGA–PVA NPs. “M” indicates the protein ladder reference, while “ctrl” is the control of the cell culture medium treated with the same procedure but without NPs.
Significance of the Results:
- The formation of the protein corona is a critical factor influencing the biological activity of nanoparticles. The results demonstrate that the surface properties of the NPs play a significant role in determining the extent and nature of the protein interactions, which directly affects their uptake by cells.
Key Innovations:
- The study introduced a novel method to quantify and characterize the protein corona in relation to different nanoparticle coatings, providing a deeper understanding of how the NP surface interacts with the biological environment.
- The use of SDS-PAGE and DLS to investigate the protein corona formation is an innovative approach to understanding how surface modifications influence NP behavior in complex biological systems.
3. Cellular Uptake Assay
Key Steps:
- Cell Incubation: The immortalized murine microglial cell line (BV2) was cultured and incubated with the 19F–PLGA NPs for 4, 6, and 8 hours. The cells were then washed to remove any unbound NPs.
- Flow Cytometry Analysis: Fluorescently labeled NPs were used to track internalization. Flow cytometry was employed to measure the fluorescence intensity, which correlates with NP uptake.
Results and Key Data:
- The cellular uptake of NPs was highest for NaC and PVA–CS-coated formulations, with NaC-coated NPs showing a 61% reduction in uptake when phagocytosis was inhibited.
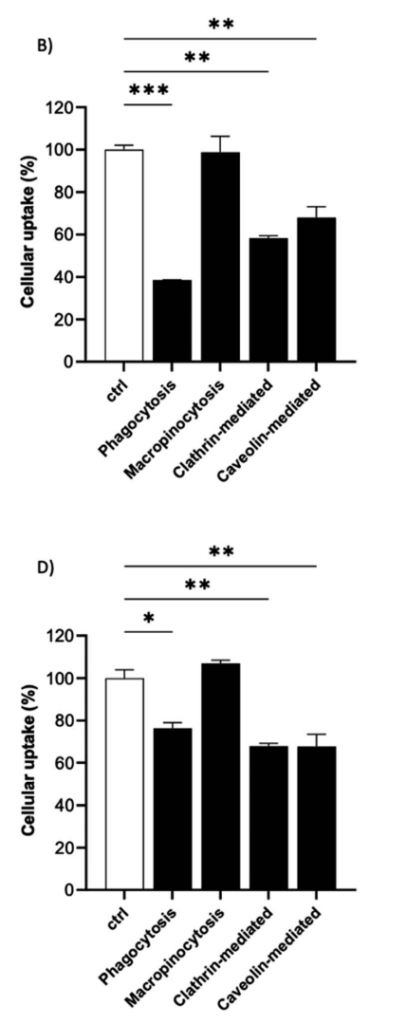
Figure 3. Preferential endocytic pathways of 19F–PLGA-NPs. The uptake of 19F–PLGA–NaC (B) and 19F–PLGA–PVA–CS (D) NPs in BV2 cells were evaluated in the presence of pharmacological inhibitors of specific endocytic route and compared to untreated labeled cells (ctrl). Data were obtained by flow cytometry in independent experiments and expressed as follows: *p < 0.05; **p < 0.01; ***p < 0.001 (one way ANOVA test compared to the ctrl)
- PVA-coated NPs exhibited the lowest uptake, reflecting the reduced ability of neutral particles to interact with cell membranes.
- Phagocytosis was identified as the primary internalization mechanism for NaC-coated NPs, while clathrin- and caveolin-mediated endocytosis played a significant role in the uptake of PVA–CS-coated NPs.
Significance of the Results:
- These findings highlight the importance of surface charge and protein corona formation in modulating nanoparticle uptake by cells. Understanding these mechanisms is crucial for optimizing NPs for targeted delivery in therapeutic applications.
Key Innovations:
- The use of flow cytometry to simultaneously assess both NP uptake and cell viability provides a comprehensive analysis of cellular interactions, improving the accuracy of NP performance evaluation.
- The innovative application of specific pharmacological inhibitors to dissect endocytic pathways offers novel insights into the cellular mechanisms governing NP internalization.
4. Magnetic Resonance Imaging (MRI) and Signal Quantification
Key Steps:
- MRI Acquisition: The 19F–PLGA NPs were imaged using 19F-MRI, a powerful technique for tracking the labeled cells. 19F-MRI was performed at four different fluorine concentrations, and the NP formulations were analyzed for their MRI signal properties.
- Quantification of Signal: Signal-to-noise ratios (SNR) were calculated for each sample to assess the efficiency of cell labeling.
Results and Key Data:
- NPs with higher 19F content and a richer protein corona produced stronger 19F signals, demonstrating the increased sensitivity of these formulations in MRI.
- The SNR of the NaC and PVA–CS-coated NPs was significantly higher compared to PVA-coated NPs, supporting the hypothesis that the protein corona enhances NP detection by 19F-MRI.
Significance of the Results:
- These results demonstrate that the 19F signal correlates with the amount of NP internalized by cells, making 19F-MRI a viable technique for monitoring cell labeling. The increased signal sensitivity of certain NP formulations could enhance the application of 19F-MRI in cell-based therapies and diagnostics.
Key Innovations:
- The innovative integration of 19F-MRI with NP formulations allows for non-invasive tracking of cells over time, a significant advancement in the field of in vivo imaging.
- The ability to quantify 19F signal in cells provides a reliable method for evaluating the efficiency of NP formulations in cell labeling, crucial for future clinical applications.
5. Flow Cytometry for Cellular Uptake Analysis
Key Steps:
- Preparation of Fluorescent NPs: To enable flow cytometry detection, the NPs were labeled with rhodamine B. The cells were incubated with these fluorescent NPs for uptake analysis.
- Flow Cytometry Evaluation: The fluorescence intensity of the cells was measured to quantify NP internalization and assess the effects of endocytic inhibitors on uptake.
Results and Key Data:
- The flow cytometry analysis showed that NaC-coated NPs had the highest fluorescence intensity, indicating superior internalization. The uptake of NPs was significantly reduced when endocytic pathways were inhibited.
Significance of the Results:
- This method provided a detailed and quantifiable approach to studying NP uptake in cells, offering insights into the optimal surface modifications for improving internalization efficiency.
Key Innovations:
- The use of fluorescently labeled NPs for flow cytometry enabled a precise, real-time analysis of cellular uptake, facilitating the development of more efficient nanoparticle-based diagnostic tools.
Conclusion
In summary, this study highlights the pivotal role of the protein corona in enhancing the cellular uptake of fluorinated nanoparticles, ultimately improving the sensitivity of ^19F-MRI. By demonstrating that nanoparticles with specific surface modifications can significantly boost labeling efficiency, the research suggests that the bio-nano interface should be a fundamental consideration in the design of imaging agents for cell tracking. While the findings are promising, the study acknowledges limitations such as the variability in protein corona formation and the impact of different biological environments on nanoparticle behavior. Future research should explore the implications of personalized protein corona dynamics in clinical applications, particularly in the labeling of therapeutic cells, to further enhance the potential of ^19F-MRI in non-invasive imaging.
Reference:
Gatti, Lodovico, et al. “Pivotal role of the protein corona in the cell uptake of fluorinated nanoparticles with increased sensitivity for 19 F-MR imaging.” Nanoscale Advances 5.14 (2023): 3749-3760.
