Editor: Nina
This study demonstrates the development of transferrin-decorated PLGA nanoparticles loaded with an organoselenium compound as a targeted drug delivery system that effectively sensitizes multidrug-resistant tumor cells, enhancing cancer therapy through increased cytotoxicity, apoptosis induction, and tumor growth inhibition.
Key Preview
Research Question
This study investigates whether transferrin-decorated PLGA nanoparticles loaded with an organoselenium compound can sensitize multidrug-resistant (MDR) tumor cells to enhance cancer therapy.
Research Design and Strategy
The research developed Tf-conjugated PLGA nanoparticles and used in vitro cytotoxicity assays with 2D and 3D cell models to assess their effectiveness against MDR tumor cells.
Method
Nanoparticles were synthesized and characterized, and their cytotoxic effects were evaluated on both sensitive and MDR tumor cells using assays to measure cell viability, migration, and apoptosis.
Key Results
The Tf-decorated nanoparticles demonstrated significantly higher antiproliferative activity and reduced cell viability in MDR cells to approximately 23.09%, with increased apoptosis and internalization compared to non-targeted nanoparticles.
Significance of the Research
This research presents a promising drug delivery system that can overcome MDR, enhancing cancer therapy by targeting tumor cells with transferrin-conjugated nanoparticles.
Introduction
The evolution of cancer treatment has seen significant advancements, yet chemotherapy remains a cornerstone of therapy. However, the emergence of multidrug resistance (MDR) poses a considerable challenge, leading to treatment failures and cancer recurrence. As such, innovative strategies to improve the effectiveness of chemotherapy are urgently needed. Recent studies have pivoted towards the utilization of nanotechnology to enhance drug delivery systems. In particular, the incorporation of transferrin as a targeting ligand capitalizes on the overexpression of transferrin receptors in tumor cells, thus allowing for more precise drug delivery. This study seeks to address the critical question: Can transferrin-decorated PLGA nanoparticles loaded with an organoselenium compound effectively sensitize MDR tumor cells? The necessity of this research stems from the pressing need to develop novel therapeutic strategies that can combat MDR and enhance cancer treatment efficacy.
Research Team and Objective
The research was conducted by a team of scholars from the Federal University of Santa Maria, Brazil, including Letícia Bueno Macedo, Daniele Rubert Nogueira-Librelotto, Daniela Mathes, and others. This study, titled “Transferrin-Decorated PLGA Nanoparticles Loaded with an Organoselenium Compound as an Innovative Approach to Sensitize MDR Tumor Cells: An In Vitro Study Using 2D and 3D Cell Models,” was published in the journal Nanomaterials in August 2023. The primary objective of the research was to develop an effective drug delivery system that not only targets tumor cells but also enhances the therapeutic efficacy against MDR tumors.
Experimental Process
Outline:
- Synthesis of Transferrin-Decorated PLGA Nanoparticles (Tf-ACAT-Se-PLGA-NPs)
- Characterization of Nanoparticles
- In Vitro Drug Release Studies
- Cytotoxicity Assay Using 2D Cell Models
- Cell Uptake and Intracellular Retention Studies
Synthesis of Transferrin-Decorated PLGA Nanoparticles (Tf-ACAT-Se-PLGA-NPs)
Key Steps: The organoselenium compound ACAT-Se was nanoencapsulated in PLGA nanoparticles (NPs) using a solvent evaporation method. The NPs were then functionalized with transferrin (Tf) by activating the surface of the NPs with carbodiimide (EDC) and conjugating Tf to the nanoparticles via a covalent bond.
Result and Key Data: The Tf-conjugated NPs exhibited a mean particle size of 137 ± 16.66 nm with a polydispersity index (PDI) of 0.127 ± 0.03, indicating a homogeneous distribution. The zeta potential of the nanoparticles was -4.36 ± 0.93 mV, and the pH was 7.38 ± 0.30. The successful conjugation of Tf was confirmed by a conjugation rate of 99.37%.
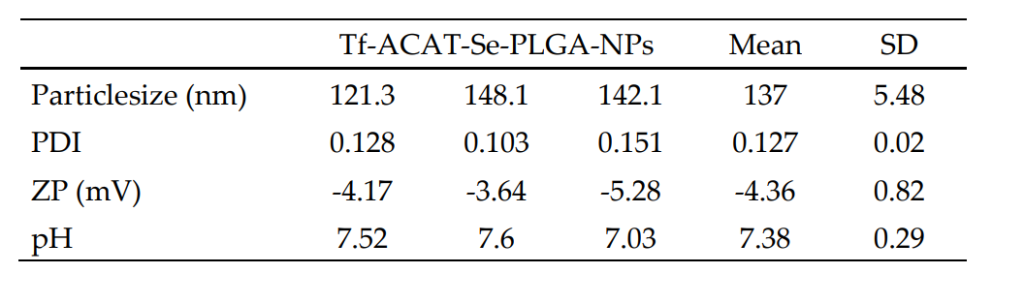
Table 1.Characterization of Tf-ACAT-Se-PLGA-NPs.
Significance of the Result: The synthesis and conjugation of Tf to PLGA NPs successfully enhanced their targeting ability toward tumor cells, as Tf is known to bind to the transferrin receptor, which is overexpressed in many cancers, particularly MDR tumor cells.
Key Innovations: The innovation lies in combining PLGA NPs with transferrin for active targeting of MDR tumor cells, alongside the inclusion of an organoselenium compound that has potential antitumor activity.
Characterization of Nanoparticles
Key Steps: The nanoparticles were characterized for their size, surface charge, morphology, and drug content. Dynamic light scattering (DLS) was used to measure hydrodynamic size and PDI, while scanning electron microscopy (SEM) provided morphological insights. The drug content and Tf conjugation were quantified by reverse-phase liquid chromatography (RP-LC) and Bradford dye-binding assay.
Result and Key Data: The NPs showed spherical morphology with a regular surface, as observed by SEM. The drug content was quantified as 1.50 ± 0.14 mg/mL, representing 78.00% of the theoretical drug content. The Tf conjugation rate was 99.37%, confirming efficient functionalization.
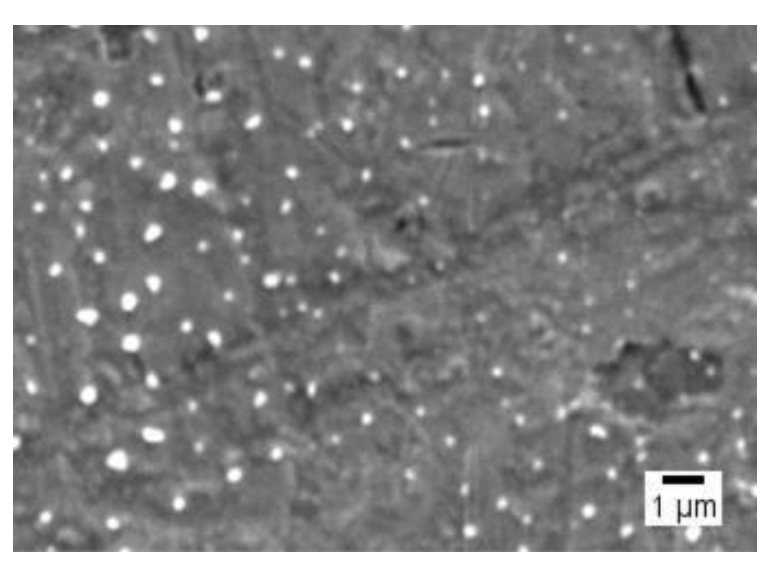
Figure 1. Tf-ACAT-Se-PLGA-NPs’ morphology by SEM.
Significance of the Result: This comprehensive characterization ensures that the nanoparticles are well-formed, stable, and effectively loaded with the organoselenium compound, with proper conjugation of Tf to enhance specificity for tumor cells.
Key Innovations: The use of both PLGA NPs and Tf as targeting agents combined with organoselenium for enhanced antitumor activity is a novel approach in overcoming MDR in cancer therapy.
In Vitro Drug Release Studies
Key Steps: The release of ACAT-Se from Tf-conjugated NPs was assessed using the dialysis method. The NPs were placed in dialysis bags and incubated in phosphate-buffered solution (PBS) at 37°C. At specific time intervals, the release of ACAT-Se was measured by RP-LC.
Result and Key Data: The release profiles showed an initial burst release of 82.67 ± 3.40% after 2 hours, followed by controlled release over 24 hours. The controlled release was linked to the encapsulation of ACAT-Se within the PLGA matrix, while the burst release was attributed to the compound on the nanoparticle surface.
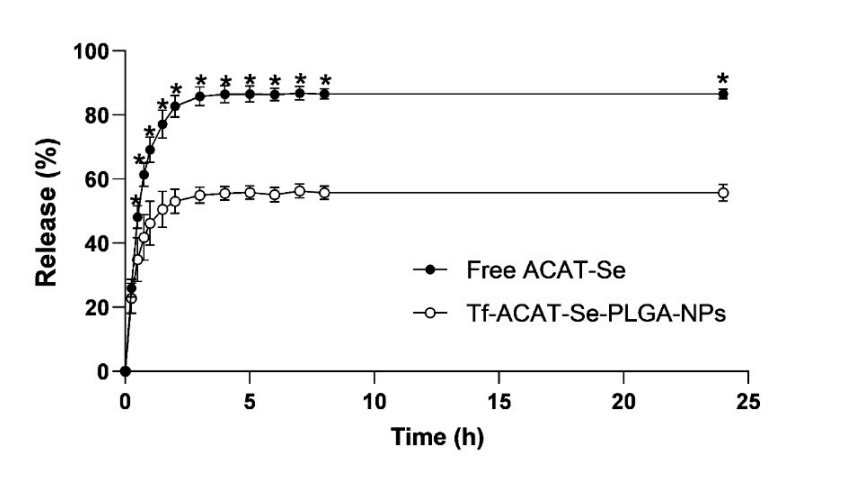
Figure 2.In vitro cumulative ACAT-Se release. Statistical analyses were performed using ANOVA followed by Tukey’s multiple comparison test. *Significant difference from Tf-ACAT-Se-PLGA-NPs (p < 0.05).
Significance of the Result: The controlled release of the drug from the nanoparticles ensures sustained delivery, which is crucial for maintaining therapeutic efficacy and minimizing side effects in cancer treatment.
Key Innovations: The combination of burst and controlled release mechanisms within a single nanoparticle formulation is an innovative strategy to optimize drug delivery and therapeutic outcomes.
Cytotoxicity Assay Using 2D Cell Models
Key Steps: Tumor cell lines (MCF-7, A375, HeLa, U-87, and NCI/ADR-RES) were treated with free ACAT-Se, ACAT-Se-PLGA-NPs, or Tf-ACAT-Se-PLGA-NPs for 24 and 72 hours. The cytotoxicity was evaluated using the MTT assay to determine cell viability.
Result and Key Data: The Tf-conjugated nanoparticles exhibited significantly greater cytotoxicity than the non-targeted nanoparticles and free ACAT-Se. For instance, after 72 hours, the Tf-NPs reduced the cell viability of NCI/ADR-RES (MDR) cells to 23.09%, compared to 91.47% with free ACAT-Se.
Significance of the Result: The enhanced cytotoxicity of the Tf-decorated NPs demonstrates the effectiveness of the targeting strategy, specifically against MDR tumor cells, where traditional chemotherapy agents often fail.
Key Innovations: The targeted approach using transferrin decoration significantly improves the therapeutic efficacy of the nanoparticles, especially in overcoming MDR, a major challenge in cancer treatment.
In Vitro Antitumor Activity Using 3D Cell Models (Tumor Spheroids)
Key Steps: 3D tumor spheroids were formed using HeLa and NCI/ADR-RES cells. The spheroids were treated with Tf-ACAT-Se-PLGA-NPs, ACAT-Se-PLGA-NPs, or free ACAT-Se for 12 days. The size reduction of the spheroids was monitored, and the area was quantified using ImageJ software.
Result and Key Data: The Tf-decorated NPs significantly inhibited spheroid growth, with a reduction in the spheroid area to 88.21% in HeLa cells and 60.93% in NCI/ADR-RES cells at the highest treatment concentration (60 µg/mL and 120 µg/mL, respectively).
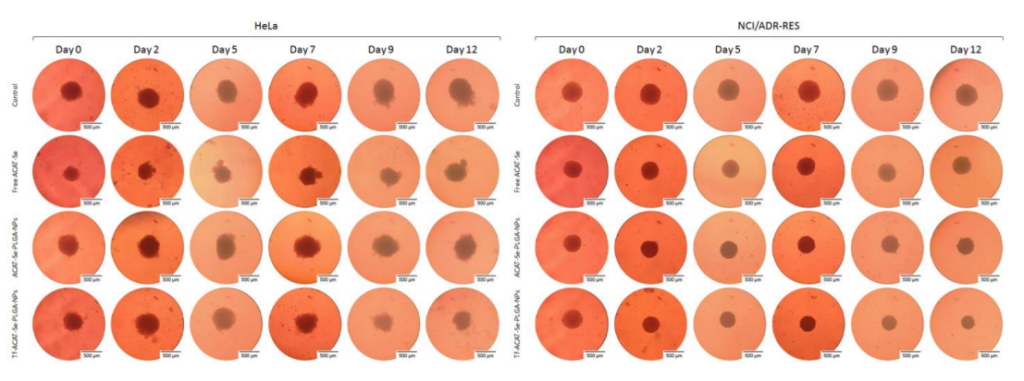
Figure 3. Representative images of spheroids treated with free ACAT-Se, ACAT-Se-PLGA-NPs or Tf-ACAT-Se-PLGA-NPs at 60 µg/mL (HeLa) or 120 µg/mL (NCI/ADR-RES). Images were obtained using inverted microscope at day 0 (before treatment), and after exposure to the treatments for 2, 5, 7, 9 and 12 days.
Significance of the Result: The 3D cell model more closely mimics the in vivo tumor microenvironment, and the enhanced antitumor effect of the Tf-conjugated NPs in this model highlights the potential of this drug delivery system for real-world therapeutic applications.
Key Innovations: The use of 3D spheroid models for testing nanoparticle efficacy provides a more accurate representation of tumor behavior compared to traditional 2D cultures, which is a critical advancement in cancer research.
Cell Migration Inhibition Assay
Key Steps: The wound-healing assay was performed to assess the ability of the nanoparticles to inhibit tumor cell migration. HeLa and NCI/ADR-RES cells were cultured to confluence, and a scratch was made on the cell monolayer. The cells were then treated with Tf-ACAT-Se-PLGA-NPs and other formulations for 48 hours.
Result and Key Data: The Tf-conjugated NPs significantly reduced cell migration in both cell lines, with migration rates of 20.46% for HeLa cells and 31.86% for NCI/ADR-RES cells, compared to 76.84% and 68.64% in the untreated controls, respectively.
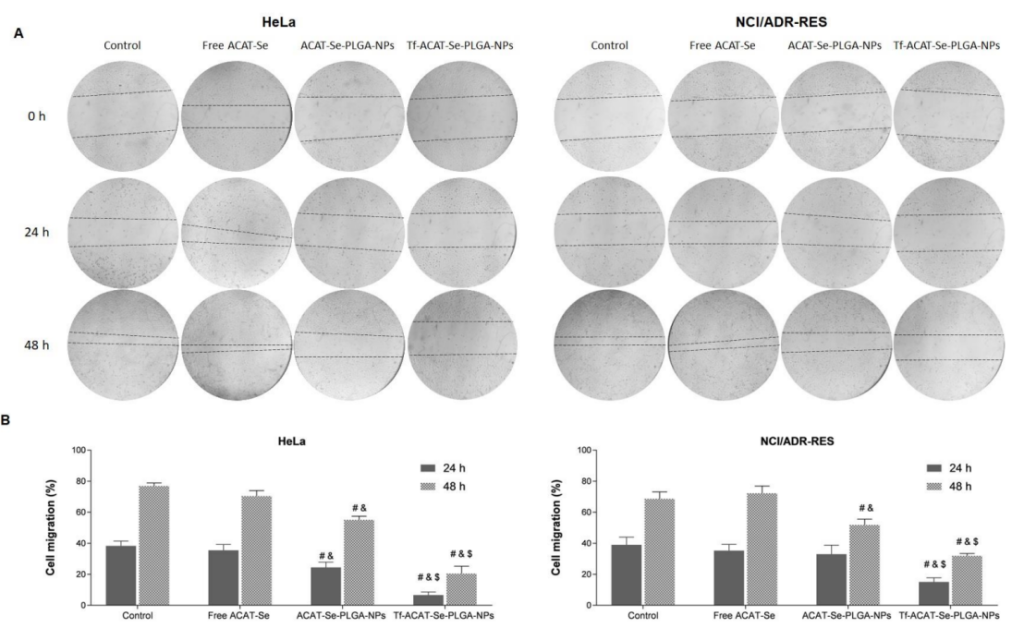
Figure 4.Effects of ACAT-Se on the motility of HeLa and NCI/ADR-Res cells. (A) Images obtained using inverted microscope at time points 0, 24 and 48 h. (B) Cell migration percentages after 24 and 48 h treatments. Statistical analyses were performed using ANOVA followed by the Student–Newman–Keuls multiple comparison test. # is different from control, & is different from free ACAT-Se and $ is different from ACAT-Se-PLGA-NPs (p < 0.05)
Significance of the Result: Inhibition of cell migration suggests that Tf-ACAT-Se-PLGA-NPs not only reduce cell proliferation but also potentially prevent metastasis, a critical factor in cancer progression.
Key Innovations: This experiment showcases the dual effect of the nanoparticles in inhibiting both tumor growth and migration, which is essential for improving the overall prognosis of cancer treatment.
Cell Uptake and Intracellular Retention Studies
Key Steps: The uptake of Tf-ACAT-Se-PLGA-NPs was assessed using flow cytometry by incubating HeLa and NCI/ADR-RES cells with rhodamine B-labeled nanoparticles. The intracellular retention of the nanoparticles was evaluated by measuring fluorescence intensity over time.
Result and Key Data: The Tf-conjugated nanoparticles were significantly more internalized by both cell lines, with a 39.5-fold higher uptake in HeLa cells and a 1.7-fold higher uptake in NCI/ADR-RES cells compared to the non-targeted NPs. Additionally, the Tf-decorated NPs exhibited superior intracellular retention after 4 hours.
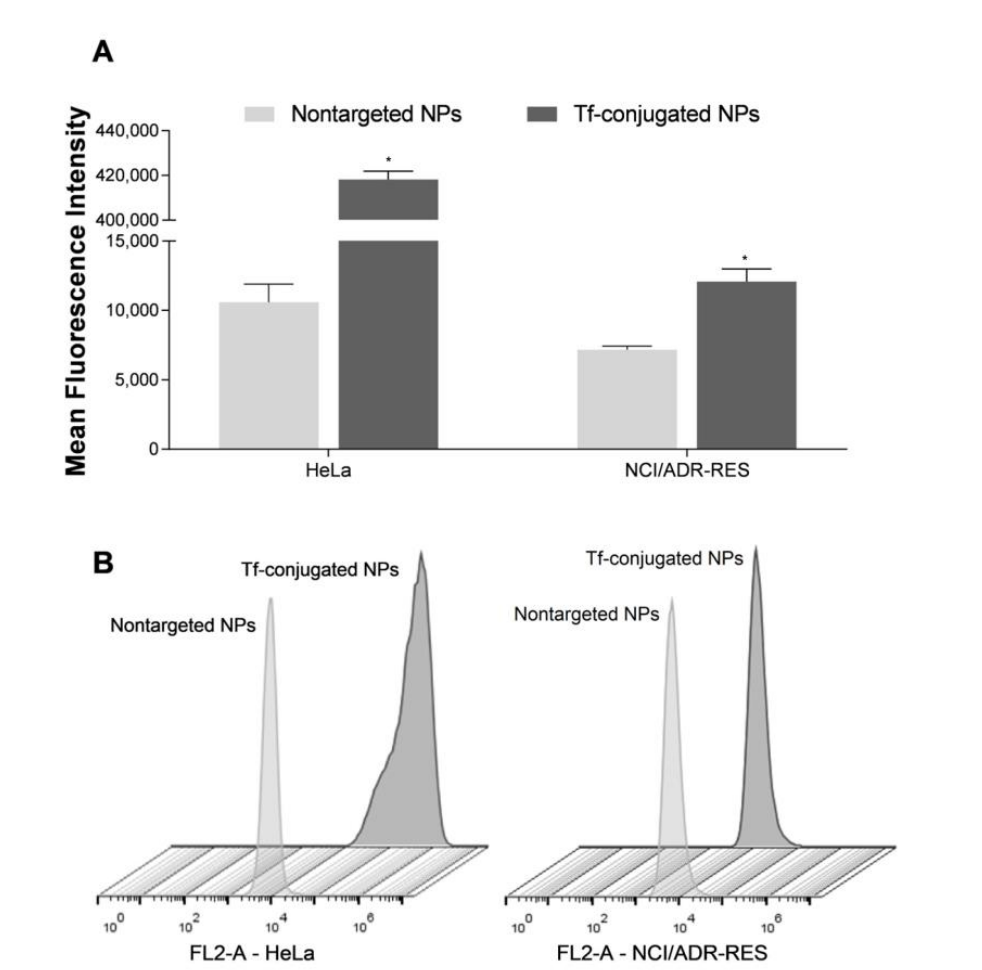
Figure 5. Cell uptake of nontargeted NPs and Tf-conjugated NPs in HeLa and NCI/ADR-RES cells. (A) Mean fluorescence intensity determined using flow cytometry. (B) Flow cytometry profiles of cellular uptake. Results are expressed as mean ± SE of three independent experiments. Statistical analyses were performed using ANOVA followed by the Student–Newman–Keuls multiple comparison test. * Significant difference from nontargeted NPs (p < 0.05)
Significance of the Result: The higher internalization and prolonged retention of the Tf-decorated NPs highlight their enhanced targeting and effectiveness in delivering the drug directly to tumor cells.
Key Innovations: The study of intracellular retention in conjunction with uptake further emphasizes the sustained activity of the Tf-conjugated nanoparticles, ensuring a more effective and long-lasting therapeutic effect.
Conclusion
The study successfully demonstrated that transferrin-decorated ACAT-Se-loaded nanoparticles significantly enhance the cytotoxicity against both sensitive and MDR tumor cells. The implications of this research are profound, suggesting that such targeted formulations could provide a viable strategy to overcome the challenges posed by multidrug resistance in cancer therapy. However, the research is not without limitations, as further investigations in vivo are necessary to validate these findings and assess the long-term efficacy and safety of the proposed treatment. Future research should focus on exploring the potential of these nanoparticles in clinical settings and evaluating their performance against a broader range of cancer types and MDR profiles.
Reference:
Macedo, Letícia Bueno, et al. “Transferrin-Decorated PLGA Nanoparticles Loaded with an Organoselenium Compound as an Innovative Approach to Sensitize MDR Tumor Cells: An In Vitro Study Using 2D and 3D Cell Models.” Nanomaterials 13.16 (2023): 2306.
