Editor: Sarah
Pancreatic cancer remains one of the most challenging cancers to treat, with poor prognosis and limited effective treatment options. Recent advances in nanotechnology and gene therapy have opened new possibilities for more precise and effective treatments. A research team led by Jiaqi Yan has made significant strides in improving photothermal therapy (PTT) for pancreatic cancer by introducing an innovative autocatalytic multicomponent DNAzyme (MNAzyme) nanomachine. This approach, detailed in a study published in Nature Communications, addresses a critical barrier in cancer treatment: the resistance induced by heat shock proteins (HSPs) during PTT. By combining gene silencing with nanomedicine, the team has developed a method to increase the sensitivity of pancreatic tumors to PTT, providing a potential path for more effective cancer treatment.
Addressing Challenges in Photothermal Therapy
Photothermal therapy works by using light to generate heat in cancerous tissues, leading to tumor cell death. However, the efficacy of PTT is often limited by the body’s natural response to heat stress—specifically, the production of heat shock proteins (HSPs). These proteins help protect cells from the damaging effects of heat, thereby reducing the effectiveness of PTT. Overcoming this protective mechanism has been a significant challenge in the field of cancer therapy.
In this groundbreaking study, the research team designed a nanomachine that targets and silences the production of HSPs. The key component of this system is the MNAzyme nanomachine, which cleaves HSP70 mRNA in tumor cells, preventing the production of these protective proteins. The system is also capable of targeting miRNA-21, an oncogene that suppresses the tumor-suppressor gene PTEN. By silencing miRNA-21, the nanomachine upregulates PTEN, which sensitizes tumor cells to PTT. This dual mechanism—silencing HSPs and upregulating PTEN—enhances the effectiveness of PTT in pancreatic cancer cells.
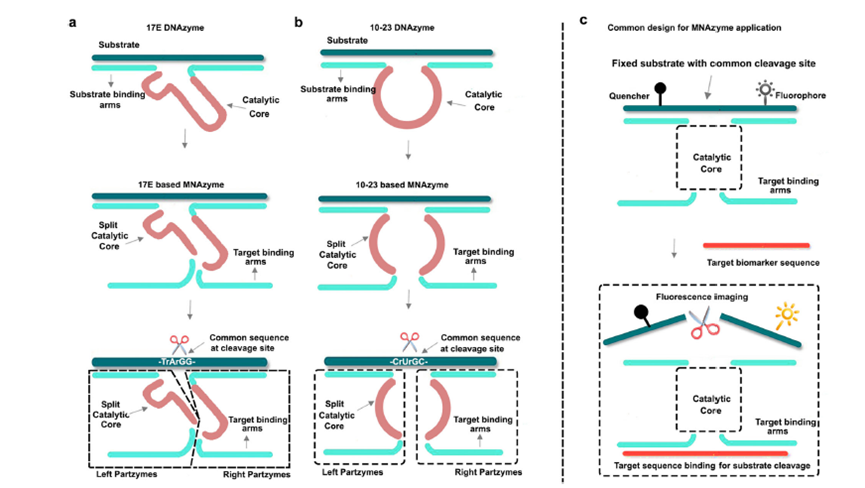
Figure 1: Structures of MNAzymes.
Key Findings and In Vivo Validation
The in vitro and in vivo experiments conducted by the research team demonstrated the effectiveness of the MNAzyme-based nanomachine. When combined with mild laser irradiation, the MNAzyme nanomachine significantly inhibited the growth of pancreatic tumors. In vitro tests showed that the system was highly effective in sensitizing tumor cells to PTT, reducing the heat resistance of the cells by silencing the HSPs.
One of the standout features of this nanomachine is its ability to selectively target tumor cells while sparing healthy tissue. In an orthotopic pancreatic tumor model, the MNAzyme system successfully targeted the tumor cells, delivering the gene-silencing therapy directly to the cancerous tissue. This targeting capability minimizes the potential side effects of treatment, a crucial aspect in cancer therapy where off-target effects can cause significant harm to healthy tissues.
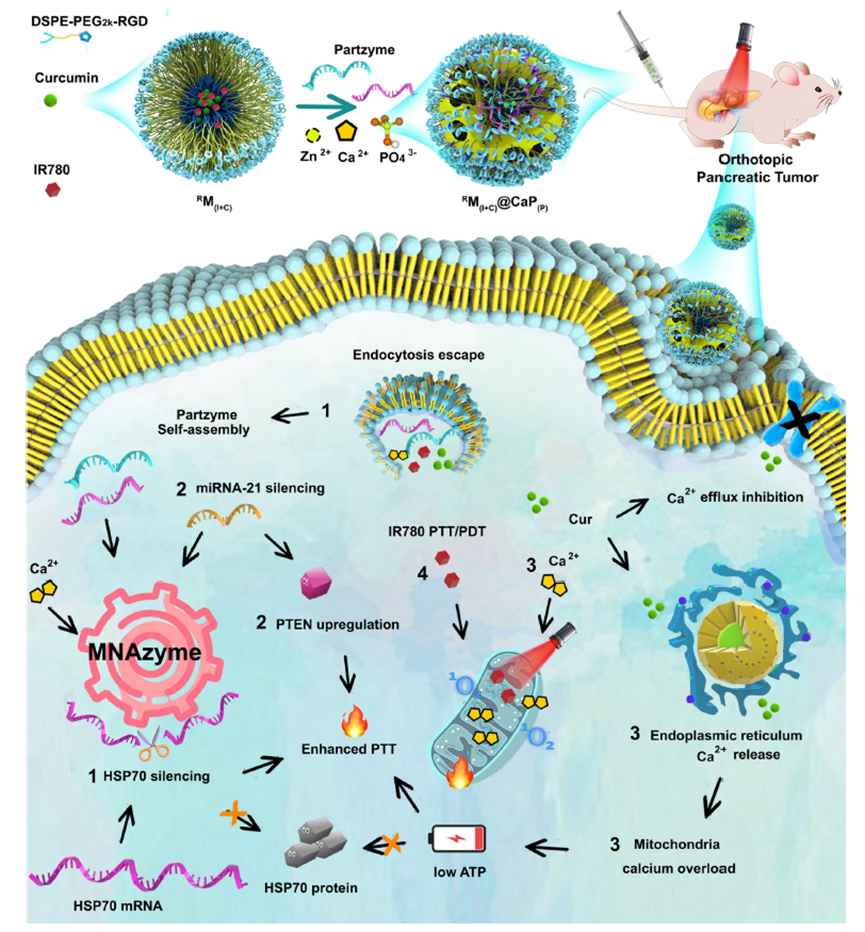
Figure 2: Illustration of four-step reactions after nanosystem fabrication and delivery.
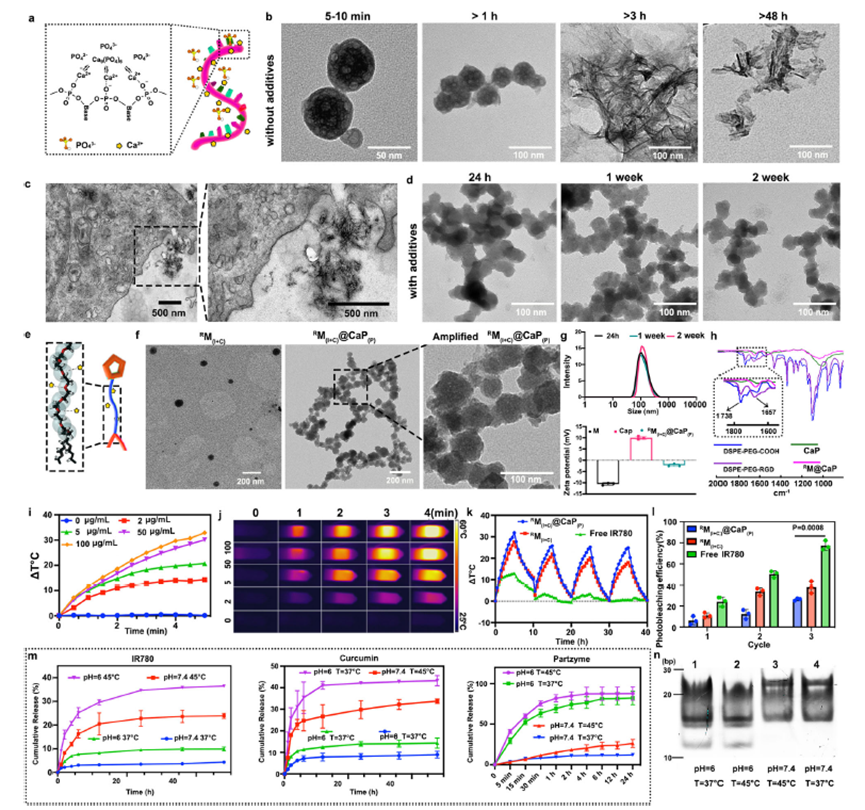
Figure 3: Construction, drug loading and photothermal characterization of the designed nanosystem.
A Multifaceted Approach to Treatment
The MNAzyme-based system works in conjunction with other therapeutic components to enhance its overall efficacy. The nanomachine is housed within a nanocarrier made from calcium phosphate (CaP) nanoparticles, which are essential for delivering the necessary cofactors for gene silencing. Additionally, the system incorporates curcumin, a compound known to maintain high intracellular calcium levels. This further sensitizes tumor cells to PTT by disrupting the mitochondria and enhancing the therapeutic effect.
The MNAzyme nanomachine is designed to achieve a multifaceted therapeutic approach. While it targets the gene regulation mechanisms involved in tumor growth, it also disrupts the mitochondria of the cancer cells. This two-pronged attack—gene silencing combined with mitochondrial disruption—further enhances the sensitivity of the tumors to PTT, providing a more effective treatment option.
Implications for the Future of Cancer Treatment
This research has significant implications for the future of cancer treatment, especially in improving the efficacy and precision of PTT for pancreatic cancer. By combining gene silencing with nanomaterial-based delivery systems, this approach offers a potential strategy for non-invasive treatments with minimal side effects. Unlike traditional treatments that often involve systemic drug administration, which can cause harm to healthy tissues, this targeted therapy focuses directly on the cancer cells, enhancing the effectiveness of the treatment.
The ability to selectively target and treat tumors also addresses the challenges of off-target effects, which have been a major limitation in gene therapy. The use of the MNAzyme-based system in combination with PTT offers a promising new avenue for overcoming these limitations, providing a more precise and effective approach to cancer treatment.
In the broader context of cancer therapy, the study provides valuable insights into overcoming the limitations of traditional treatments. By offering a strategy that combines gene silencing, photothermal therapy, and nanomedicine, the research paves the way for more effective and less invasive treatments. This approach could be particularly beneficial for patients with pancreatic cancer, a disease that has limited treatment options and poor survival rates.
A Step Toward Personalized Cancer Therapy
The successful development of the MNAzyme-based system highlights the potential for personalized cancer therapies. By targeting specific genes and using nanocarriers to deliver therapeutic agents directly to the tumor site, this approach could be adapted to treat a variety of cancers with similar gene expression profiles. The ability to tailor treatments to the specific genetic makeup of each tumor could significantly improve the success rates of cancer therapies.
Furthermore, the system’s ability to target and regulate specific genes, such as miRNA-21 and HSP70, demonstrates the potential for using gene therapy in conjunction with other therapeutic modalities to achieve a more comprehensive treatment effect. This opens up exciting possibilities for the future of cancer treatment, where personalized, targeted therapies could replace traditional, one-size-fits-all approaches.
Conclusion
In summary, the development of the MNAzyme nanomachine represents a significant advancement in the field of cancer therapy, particularly for pancreatic cancer. By overcoming the heat shock protein-induced resistance to photothermal therapy and enhancing the sensitivity of tumor cells to PTT, this innovative approach offers new hope for patients with limited treatment options. With its ability to combine gene silencing, nanomedicine, and photothermal therapy, this approach could transform the landscape of cancer treatment and provide a more effective, targeted alternative to traditional therapies. As research continues, the promise of personalized, non-invasive cancer treatments that minimize side effects and improve patient outcomes may become a reality.
Reference
Yan, Jiaqi, et al. “An Autocatalytic Multicomponent DNAzyme Nanomachine for Tumor-Specific Photothermal Therapy Sensitization in Pancreatic Cancer.” Nature Communications, vol. 14, no. 6905, 2023, doi:10.1038/s41467-023-42740-2.
