Editor: Sarah
Influenza remains a persistent global health challenge, with seasonal epidemics causing widespread illness and significant mortality. Addressing these challenges, researchers at the Vaccine Research Center (VRC) of the National Institute of Allergy and Infectious Diseases (NIAID) have developed an innovative influenza vaccine named H1ssF. Unlike conventional vaccines, which focus on the hemagglutinin (HA) head prone to antigenic drift, this new vaccine targets the conserved HA stem region, offering potential for broad-spectrum and long-lasting protection. The first-in-human phase 1 clinical trial of H1ssF represents a promising step toward achieving a universal influenza vaccine.
Key Findings
The research team employed ferritin nanoparticle technology to precisely present the hemagglutinin (HA) stem to the immune system. Ferritin nanoparticles, a well-established platform for vaccine development, were chosen for their ability to closely mimic the structure of the HA stem while enhancing immune recognition. By presenting the HA stem in a way that bypasses the immunodominant head region, the vaccine stimulates a robust immune response against a part of the virus that is conserved across various strains, potentially offering protection against a wide range of influenza viruses.
The findings from the phase 1 trial of the H1ssF vaccine are notable. The trial demonstrated several key results:
- Cross-Reactive Immune Responses: The vaccine successfully induced cross-reactive, neutralizing antibodies against group 1 influenza viruses in healthy adults, regardless of their previous exposure to the influenza virus. This cross-reactivity is particularly important because it suggests that the vaccine can provide protection not only against specific strains but across different influenza subtypes, an essential feature for a universal influenza vaccine.
- Safety Profile: The vaccine showed a strong safety profile with no severe adverse events reported. Common side effects were mild and localized, including tenderness at the injection site. Systemic effects such as headaches were also reported but were generally mild and short-lived. These results are crucial for the widespread acceptance and use of the vaccine, as safety is a primary concern for any new vaccine.
- Durability of Immune Response: One of the most significant findings was the durability of the immune response. Neutralizing antibodies induced by the vaccine persisted for more than a year post-vaccination. This long-lasting immunity is a promising feature for influenza vaccines, as it could reduce the need for annual vaccinations and provide sustained protection against influenza. Furthermore, a sixfold increase in stem-binding antibodies was observed after the initial dose, with neutralization titers remaining elevated at week 68. This indicates that the vaccine could provide lasting protection, even against strains of the virus that have not been directly targeted.
These findings underscore the potential of the H1ssF vaccine to provide long-term, broad-spectrum protection against multiple influenza strains. The ability to generate a durable immune response against a highly conserved region of the virus could be a game-changer in the ongoing battle against influenza.
Methodology and Implications
Key Methodological Details:
- Study Design:
- Phase 1, open-label, dose-escalation clinical trial.
- 52 healthy adults aged 18–70 participated.
- Participants received either:
- A single 20 μg dose.
- Two 60 μg doses administered 16 weeks apart.
- Safety assessments and antibody response measurements were conducted over a year.
- Vaccination Procedure:
- The H1ssF vaccine was delivered intramuscularly, targeting the deltoid muscle.
- Immune responses were measured using binding and neutralization assays.
Clinical Implications:
- Broad Protection: By focusing on the conserved HA stem, the H1ssF vaccine could address a range of influenza strains, including those not yet circulating in humans.
- Elderly and Vulnerable Populations: The vaccine’s design may enhance efficacy for populations that traditionally respond poorly to conventional vaccines, such as older adults.
- Pandemic Preparedness: Long-lasting immunity and broad coverage reduce the need for frequent reformulation, accelerating vaccine production during pandemics.
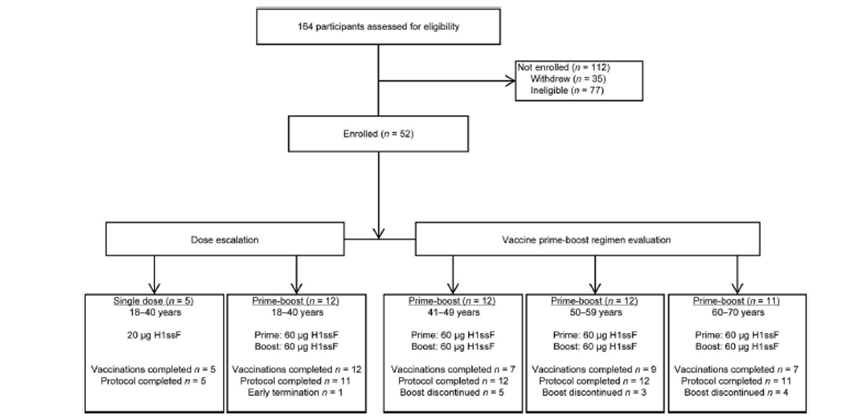
Fig. 1. Study CONSORT diagram
Conclusion
The H1ssF vaccine trial results mark a significant advancement in influenza prevention. Its novel nanoparticle design, safety, and efficacy lay the groundwork for broader applications in public health, including pandemic preparedness. While additional trials are required to validate these findings, the H1ssF vaccine holds promise for revolutionizing influenza vaccine strategies and improving global health outcomes.
Reference
Widge, Alicia T., et al. “An Influenza Hemagglutinin Stem Nanoparticle Vaccine Induces Cross-Group 1 Neutralizing Antibodies in Healthy Adults.” Science Translational Medicine, vol. 15, no. 692, 19 Apr. 2023, eade4790. doi:10.1126/scitranslmed.ade4790.
