Researchers have identified exosomal circCABIN1 as a key factor contributing to temozolomide resistance in glioblastoma, offering potential pathways for improving treatment strategies.
Key Highlights:
- Research Question:
Does exosomal circCABIN1 drive temozolomide (TMZ) resistance in glioblastoma (GBM), and can targeting circCABIN1 overcome this resistance? - Research Difficulties:
Identifying circCABIN1’s role in resistance and delivering siRNA efficiently using engineered exosomes. - Key Findings:
circCABIN1 promotes TMZ resistance via the miR-637/OLFML3/ErbB axis; targeting circCABIN1 with engineered exosomes reduced tumor volume by 68% and extended survival in mouse models. - Innovative Aspects:
First to reveal circCABIN1’s role in TMZ resistance and use engineered exosomes for targeted molecular therapy in GBM. - Importance of the Study:
Offers a new therapeutic strategy to address TMZ resistance, improving treatment options for GBM patients.
Challenges in Treating Glioblastoma
Glioblastoma Overview
Glioblastoma (GBM) is the most prevalent and lethal primary brain tumor in adults. It progresses rapidly, recurs frequently, and offers patients a median survival of only 14.6 months after diagnosis. Despite existing therapies, achieving long-term remission remains elusive.
Symptoms and Current Treatments
GBM commonly manifests as persistent headaches, seizures, and cognitive or motor impairments. Treatment often combines surgery, radiotherapy, and chemotherapy. Temozolomide (TMZ), due to its ability to cross the blood-brain barrier, is the chemotherapy of choice. However, its efficacy is significantly hindered by acquired resistance.
The Problem of Resistance
TMZ resistance is a formidable challenge in GBM therapy, arising from complex molecular mechanisms that enable tumor cells to evade treatment. Addressing this resistance is critical to improving clinical outcomes.
Objectives of the Research
This research aimed to examine the role of exosome-mediated circCABIN1 in conferring TMZ resistance in GBM and evaluate potential strategies for targeting this mechanism. The objectives included:
- Identifying the molecular processes by which circCABIN1 contributes to resistance.
- Analyzing its interaction with miR-637 and downstream signaling pathways.
- Investigating engineered exosomes as a therapeutic tool.
The study was conducted by a team from the Fourth Military Medical University, Xi’an, China, and published in the Journal of Nanobiotechnology in 2023.
Methods and Findings
Sequential Experimental Process
- Developed TMZ-resistant GBM cells through iterative TMZ exposure and culture.
- Isolated and characterized exosomes from resistant and sensitive GBM cells.
- Conducted RNA sequencing to identify circRNAs implicated in resistance.
- Validated the role of circCABIN1 in resistance through in vitro and in vivo models.
- Explored interactions between circCABIN1, miR-637, and downstream targets.
- Evaluated the therapeutic potential of engineered exosomes targeting circCABIN1.
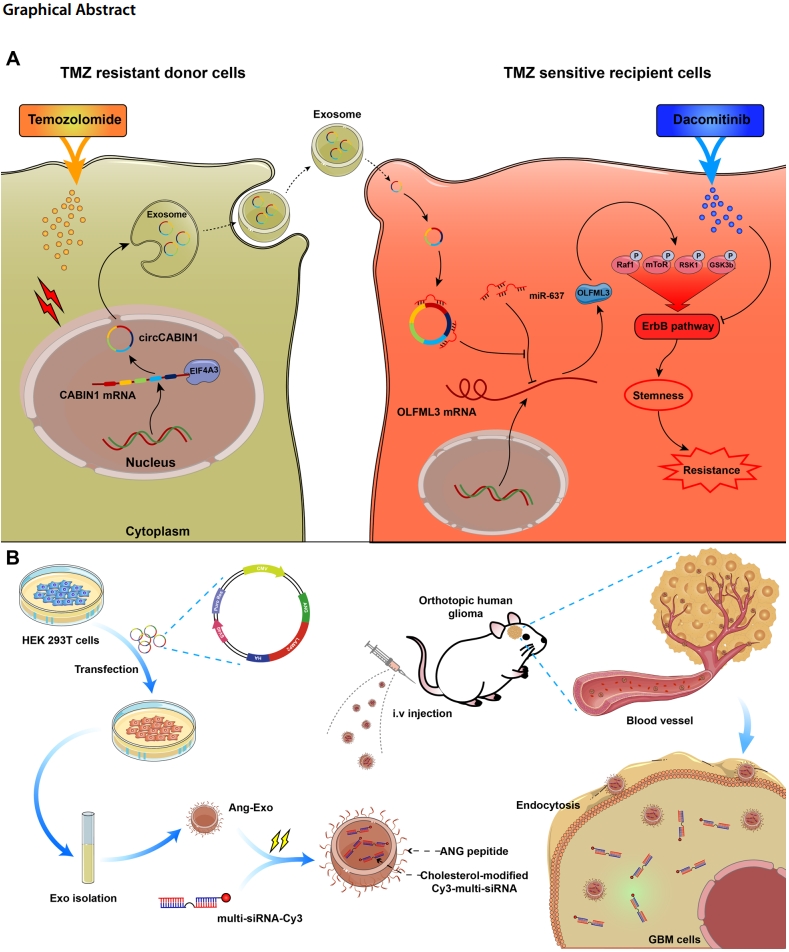
Key Experiments and Findings
a. Identification of circCABIN1 as a Resistance Mediator
Preparatory Work: Exosomes from resistant and parental GBM cells were isolated, and their properties were examined using nanoparticle tracking analysis and fluorescence imaging.
Experimental Procedure: RNA sequencing revealed that circCABIN1 was significantly upregulated in resistant cells and their exosomes. Its circular nature was confirmed through RT-PCR and Sanger sequencing.
Results: Knockdown of circCABIN1 in resistant cells reduced resistance in co-cultured parental cells, indicating its critical role in resistance transfer.
b. Mechanistic Study of the circCABIN1/miR-637/OLFML3 Axis
Preparatory Work: Researchers employed fluorescence in situ hybridization (FISH) to localize circCABIN1 and miR-637, along with luciferase assays to validate their interaction.
Experimental Procedure: circCABIN1 was shown to act as a molecular sponge for miR-637, thereby upregulating OLFML3 expression. Further analyses revealed that OLFML3 activated ErbB signaling, which sustained glioblastoma stem cell characteristics and contributed to resistance.
Results: circCABIN1 knockdown led to decreased OLFML3 levels, downregulation of cancer stemness markers (e.g., CD133, SOX2), and enhanced TMZ sensitivity in vitro and in vivo.
c. Therapeutic Application of Engineered Exosomes
Preparatory Work: Engineered exosomes were loaded with siRNA targeting circCABIN1 and tested for delivery efficiency.
Experimental Procedure: These exosomes were administered to tumor-bearing mice, and their distribution and therapeutic effects were monitored.
Results: The engineered exosomes effectively reduced tumor growth, enhanced TMZ efficacy, and improved survival outcomes in mice, demonstrating their therapeutic promise.
Summary of Insights and Implications
The study identified circCABIN1 as a pivotal element in TMZ resistance, mediating its effects through interactions with miR-637 and activation of the ErbB signaling pathway. Exosomes serve as vehicles for transmitting this resistance within tumors. By targeting circCABIN1 with engineered exosomes, the research provides a promising approach for reversing resistance and enhancing the efficacy of existing GBM therapies. These findings lay the groundwork for future investigations into exosome-mediated drug resistance, offering hope for improved treatments in one of the most challenging cancers to manage.
Reference:
Liu, Xiao, et al. “Exosome-transmitted circCABIN1 promotes temozolomide resistance in glioblastoma via sustaining ErbB downstream signaling.” Journal of Nanobiotechnology 21.1 (2023): 45.
