Editor:Nina
This study presents a novel pH-responsive nanomedicine that co-delivers a calcium channel inhibitor (TTA-Q6) and a CD47 inhibitor (RRX-001) to reprogram the tumor microenvironment, activating both innate and adaptive immune responses for enhanced lung cancer immunotherapy.
Key Preview
Research Question
- How can a novel pH-responsive nanomedicine activate anti-tumoral macrophages and dendritic cells to enhance the efficacy of lung cancer immunotherapy by overcoming the challenges posed by pro-tumoral macrophages?
Research Design and Strategy
- Developed a layered double hydroxide (LDH) nanosheet nanomedicine to co-deliver TTA-Q6 (a T-type calcium channel inhibitor) and RRX-001 (a CD47 inhibitor) into the acidic tumor microenvironment to simultaneously disrupt cancer cell signaling and reprogram immune responses.
Method
- Employed in vitro and in vivo models using male mice to evaluate the efficacy of the nanomedicine. Key steps included nanocarrier synthesis and characterization, drug release profiling, and immune activation assessments.
Key Results
- The dual-drug nanomedicine achieved a significant reduction in tumor growth, with 83% of treated mice surviving beyond three months. Enhanced calreticulin expression and suppressed CD47 levels promoted robust macrophage activation and T-cell-mediated immune responses.
Significance of the Research
- Demonstrated a novel immunotherapy strategy that combines innate and adaptive immune reprogramming, providing a promising avenue for treating solid tumors like lung cancer and addressing limitations of current therapies.
Introduction
The evolution of cancer immunotherapy has transformed treatment approaches, yet challenges remain, particularly in solid tumors like lung cancer, where an immunosuppressive microenvironment often prevails. Recent advances have highlighted the potential of targeting innate immune responses, particularly macrophages, to enhance anti-tumor immunity. The current study introduces a novel pH-responsive nanomedicine aiming to address these challenges by co-delivering a calcium channel inhibitor and a CD47 inhibitor to activate both macrophages and dendritic cells, ultimately enhancing the immune response against lung tumors. This research is crucial as it seeks to develop effective strategies to overcome the immunosuppressive barriers hindering successful lung cancer treatment.
Research Team and Objective
The research was conducted by a team led by Yuedong Guo, alongside Qunqun Bao, Ping Hu, and Jianlin Shi, at the Shanghai Institute of Ceramics, Chinese Academy of Sciences, and Shanghai Tenth People’s Hospital. The study, titled “Nanomedicine-based co-delivery of a calcium channel inhibitor and a small molecule targeting CD47 for lung cancer immunotherapy,” has been published in Nature Communications. The primary objective of this research is to explore a combined therapeutic strategy that could significantly improve the efficacy of lung cancer treatments by reprogramming the tumor immune microenvironment.
Experimental Process
Outline:
- Synthesis and Characterization of LRT Nanomedicines
- Regulation of Cancer Cell Membrane Proteins
- Activation of Macrophages and Dendritic Cells
- In Vivo Lung Cancer Immunotherapy
1. Synthesis and Characterization of LRT Nanomedicines
Key Steps:
- Synthesis of Zn-Al LDH Nanosheets:
Zn-Al LDH nanosheets were synthesized via a homogeneous alkalization method. The process involved maintaining specific conditions to ensure the nanosheets formed with appropriate size and morphology. The nanosheets were then modified with carboxyl PEG-trimethyl silane through hydrolysis of silane coupling agents, which enhanced their biocompatibility and stability. - Drug Loading:
The nanocarriers were loaded with two small-molecule drugs: TTA-Q6 (a T-type calcium channel inhibitor) and RRX-001 (a CD47 inhibitor). The drugs were intercalated into the layered structure of the nanosheets to enable controlled, pH-sensitive release. - Characterization:
The physical and chemical properties of the nanosheets were characterized using TEM, SEM, XRD, and FTIR to confirm successful synthesis, morphology, and drug encapsulation. The zeta potential of the nanosheets was also measured to verify PEGylation and surface charge.
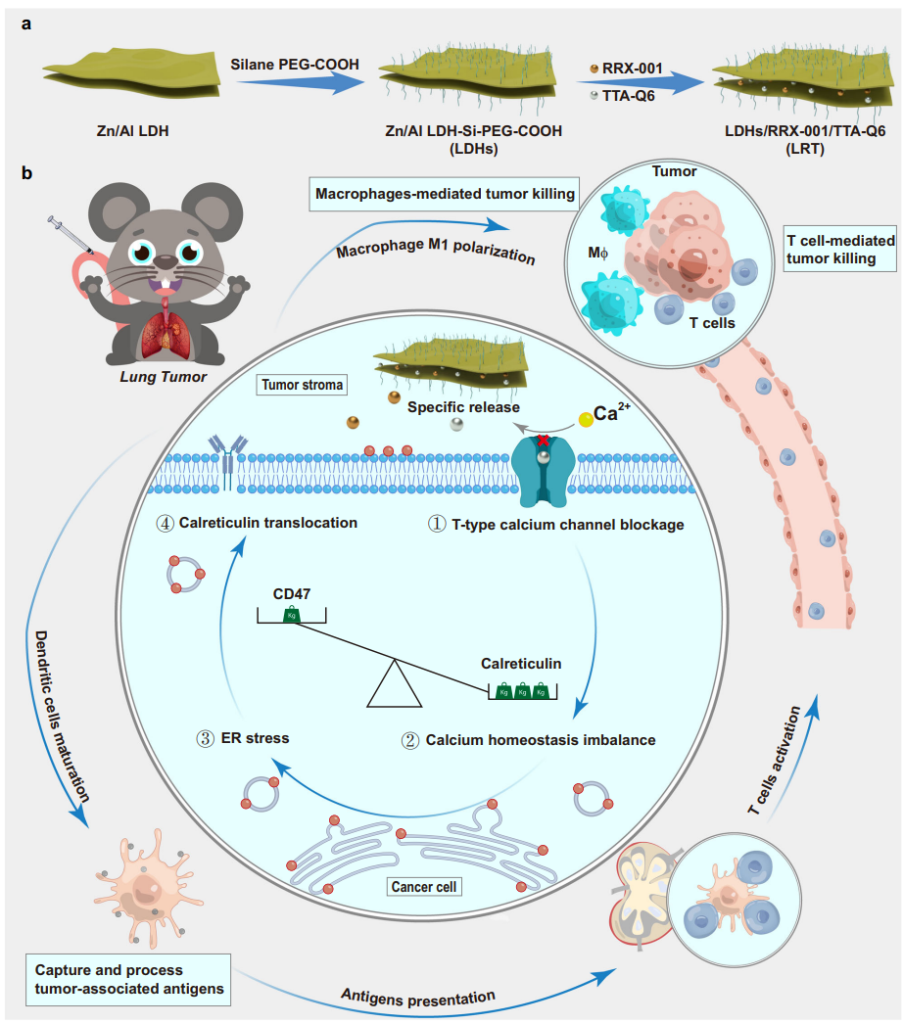
Figure 1. Schematic illustration of synthetic procedures and therapeutic mechanisms of LRT nanomedicine. a Schematic diagram of LDH nanosheet synthesis and the subsequent drug loading. b Mechanism of antitumor immune responses against orthotopic lung tumors by TTA-Q6/RRX-001 co-delivery. (1) T-type calcium influx channel blockage by TTA-Q6, (2) calcium homeostatic deficiency inparallel with theCD47 downregulation by RRX-001,(3) ER stress induction due to the calcium deficiency, (4) calreticulin translocation towards the cell membrane. MΦ represents macrophages
Results and Key Data:
- TEM and SEM images confirmed hexagonal nanosheets with a lateral dimension of ~800–900 nm and a thickness of ~40 nm.
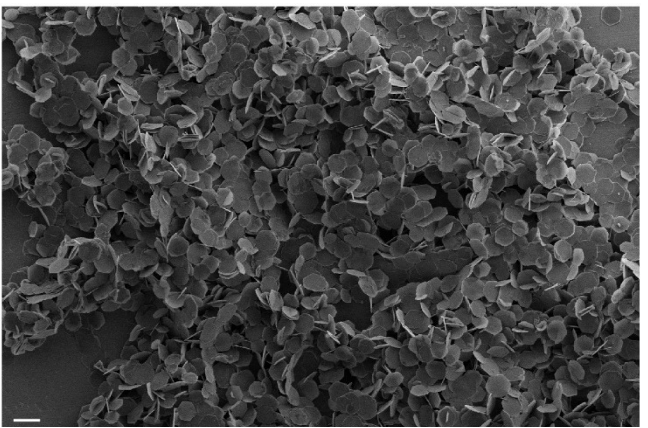
Figure 2. SEM image of LDH-PEG nanosheets, scale bar: 1 μm. A representative image of three independent samples is shown.
- XRD patterns demonstrated an increased basal d-spacing after drug intercalation, verifying successful loading.
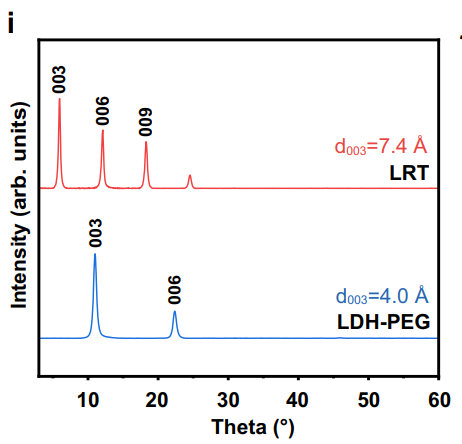
Figure 3. Powder XRD patterns of LDH nanosheets and LRT nanomedicines
- Drug release profiles showed a rapid release in acidic conditions (pH 6.5) with sustained release over 24 hours, achieving 9.7 wt% RRX-001 and 6.4 wt% TTA-Q6 released.
Significance of the Results:
- The controlled, pH-responsive drug release minimizes off-target effects and enhances drug delivery specifically to the tumor microenvironment.
- Successful synthesis and characterization of the nanocarriers confirm their suitability for co-delivery of therapeutic agents.
Key Innovations:
- Development of a dual-drug, pH-responsive nanomedicine for targeted delivery in acidic tumor environments.
- Integration of PEGylation improved biocompatibility and enhanced stability in biological systems.
2. Regulation of Cancer Cell Membrane Proteins
Key Steps:
- Nanocarrier Uptake Analysis:
The nanocarrier’s uptake by Lewis lung carcinoma (LLC) cells was evaluated using flow cytometry to confirm selective targeting of the tumor stroma. - Calcium Channel Inhibition:
Intracellular calcium levels were assessed using Calbryte 520 fluorescent indicators and confocal microscopy to demonstrate the effect of TTA-Q6 in blocking calcium influx. - Calreticulin (CRT) Translocation and CD47 Downregulation:
Flow cytometry and CLSM were used to measure the CRT exposure on the cell surface and the downregulation of CD47 on cancer cells treated with LRT.
Results and Key Data:
- Nanocarrier uptake by LLC cells was minimal (<10%), confirming selective targeting of the tumor stroma.
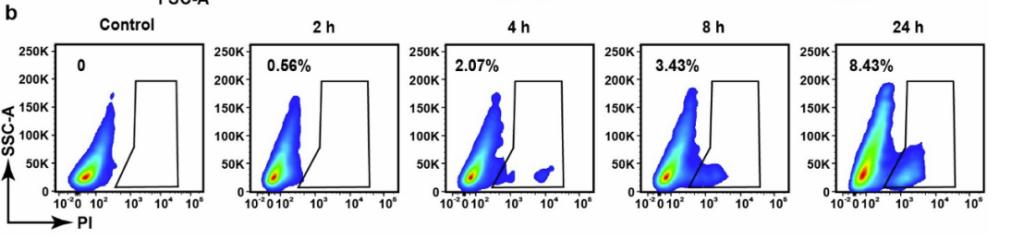
Figure 4. (b) Flow cytometry analysis of phagocytosis of LRT by LLC cells
- Calcium influx was significantly inhibited in treated cells, with reduced intracellular calcium fluorescence signals.
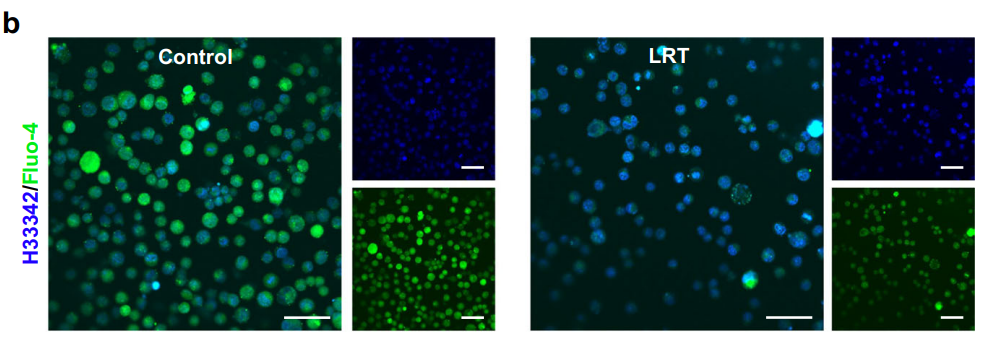
Figure 5. CLSM images of LLC cells after different treatments for 24 h. Fluo-4 (green) was used for cellular calcium observation and the nucleus were stained with Hoechst 33342(blue). Scale bars, 50 μm. N = 3 samples with similar results.
- CRT exposure was significantly upregulated, and CD47 expression was downregulated on cancer cell surfaces, marking the cells for immune recognition.
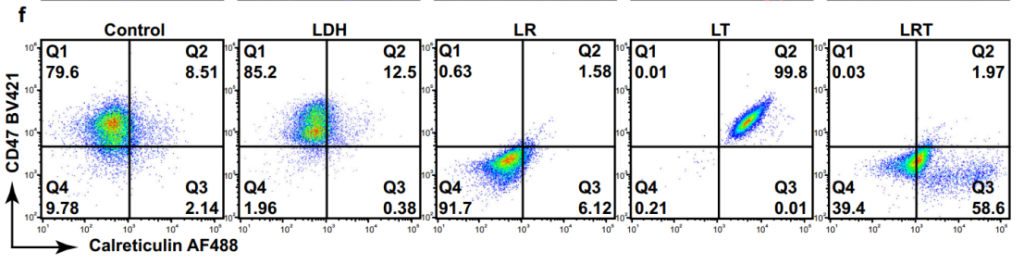
Figure 6. Flow cytometric analyses for CD47 and CRT regulations of LLC cells after various treatments, representative of 3 independent experiments.
Significance of the Results:
- CRT translocation acts as an “eat-me” signal, enhancing macrophage-mediated clearance of cancer cells.
- Downregulation of CD47 prevents immune evasion, ensuring effective immune targeting.
Key Innovations:
- Dual modulation of CRT and CD47 on cancer cell surfaces to synergistically enhance immune system activation.
- Use of a targeted nanomedicine to achieve selective and effective immune modulation.
3. Activation of Macrophages and Dendritic Cells
Key Steps:
- Macrophage Polarization:
Bone marrow-derived macrophages (BMDMs) were co-cultured with treated LLC cells to evaluate M1 polarization using cytokine profiling and morphological analysis. - Dendritic Cell Maturation:
Bone marrow-derived dendritic cells (BMDCs) were exposed to treated LLC cells to assess maturation via cytokine secretion and surface marker expression (CD80/CD86).
Results and Key Data:
- LRT-treated macrophages displayed an elongated morphology typical of M1 polarization, with elevated secretion of TNF-α, IL-6, and IL-1β.
- BMDCs treated with LRT-conditioned media showed upregulation of CD80/CD86 and increased secretion of IL-12p70, confirming maturation.
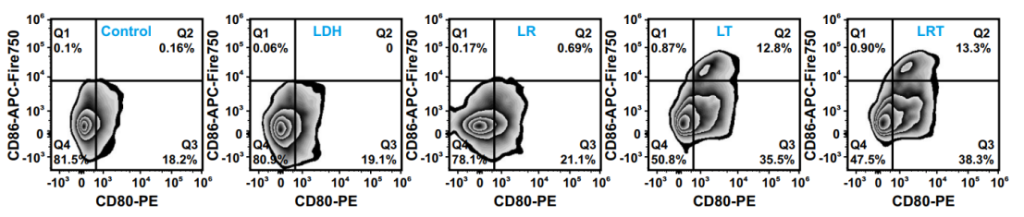
Figure 7. FACS plots of CD80 and CD86 expressions on BMDCs gated on CD11c+ cells, representative of 3 independent experiments
Significance of the Results:
- M1 macrophages enhance antitumor immunity by secreting pro-inflammatory cytokines and facilitating tumor cell phagocytosis.
- Mature DCs are critical for antigen presentation and activation of T cells, enabling robust adaptive immune responses.
Key Innovations:
- Simultaneous activation of innate and adaptive immunity using a single therapeutic platform.
- Novel combination of immune modulation strategies targeting macrophages and dendritic cells.
4. In Vivo Lung Cancer Immunotherapy
Key Steps:
- Orthotopic Tumor Model:
An orthotopic lung cancer model was established in mice by injecting LLC-Luc cells into the left lung. - Therapeutic Evaluation:
Mice were treated with PBS, LDH, LR (LDH/RRX-001), LT (LDH/TTA-Q6), or LRT. Tumor growth was monitored using micro-CT imaging and 3D lung reconstruction. - Immune Profiling:
Tumor-infiltrating immune cells were analyzed using flow cytometry to assess the activation of TAMs, DCs, and T cells.
Results and Key Data:
- LRT treatment suppressed tumor growth significantly, with 83% of mice surviving for three months.
- Immune profiling revealed increased TAM-M1, mature DCs, and CD8+ T-cell infiltration in tumors.
- Elevated levels of TNF-α and IL-12p70 in serum indicated a strong systemic immune response.
Significance of the Results:
- Demonstrates the potential of LRT nanomedicine for effective and durable lung cancer immunotherapy.
- Highlights the synergy of innate and adaptive immune activation in achieving sustained antitumor effects.
Key Innovations:
- Integration of immune-targeting strategies into a single, pH-responsive nanomedicine.
- Enhanced tumor accumulation and immune activation compared to conventional therapies.
Conclusion
The study’s findings reveal that the novel LRT nanomedicine can effectively activate anti-tumor immune responses in lung cancer models. The results indicate that this therapy not only promotes the direct killing of tumor cells but also establishes a long-term immune memory, making it a promising candidate for future lung cancer immunotherapy. Future research directions may include optimizing the nanomedicine formulation and exploring its applications in other tumor types to broaden the therapeutic potential of this innovative approach.
Reference:
Guo, Yuedong, et al. “Nanomedicine-based co-delivery of a calcium channel inhibitor and a small molecule targeting CD47 for lung cancer immunotherapy.” Nature Communications 14.1 (2023): 7306.
