Editor: Sarah
Could a new material redefine how we treat infected wounds?
Researchers from Xi’an Jiaotong University have developed a bacteria-responsive, self-activating antibacterial hydrogel that offers a promising solution for treating infected wounds and improving healing. Published in the National Science Review, this innovation addresses challenges posed by chronic skin wounds and drug-resistant bacterial infections, which have long been a global healthcare burden.
Traditional treatments often struggle to overcome bacterial resistance and biofilm formation, prolonging the healing process. This new hydrogel incorporates advanced material science to dynamically interact with bacterial microenvironments, targeting infection while fostering tissue repair.
A Smart Solution to a Persistent Problem
At the core of this technology is a multi-functional hydrogel platform designed to both eradicate bacteria and optimize the healing environment. The hydrogel uses a combination of pH-sensitive components and self-activating mechanisms to respond directly to bacterial activity. Here’s how it works:
- Targeted Antibacterial Efficiency: Laboratory testing showed that the hydrogel could eliminate 99.2% of methicillin-resistant Staphylococcus aureus (MRSA), a common drug-resistant bacterium. This was achieved through the hydrogel’s ability to break down biofilms—dense bacterial colonies that shield infections from conventional antibiotics.
- Enhanced Wound Healing: In animal models, wounds treated with this hydrogel demonstrated near-complete closure within 15 days. This represents a significant improvement over existing methods, particularly for chronic or motion-affected wounds.
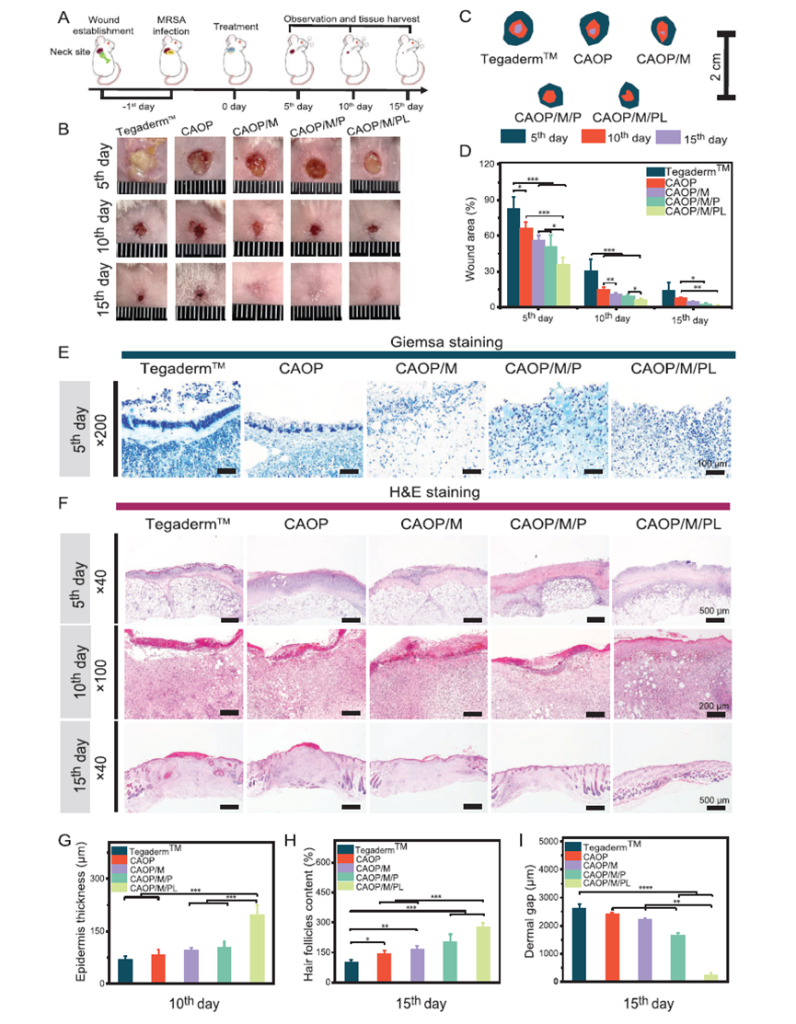
Figure 1: Photographic and quantitative analysis of wound healing in a mouse model treated with the hydrogel, demonstrating nearly complete wound closure within 15 days compared to other treatments.
- Biofilm Eradication: Biofilms are a persistent obstacle in wound treatment, often requiring invasive removal. The hydrogel reduced biofilm levels by over 80%, thanks to its ability to disrupt bacterial colonies dynamically.
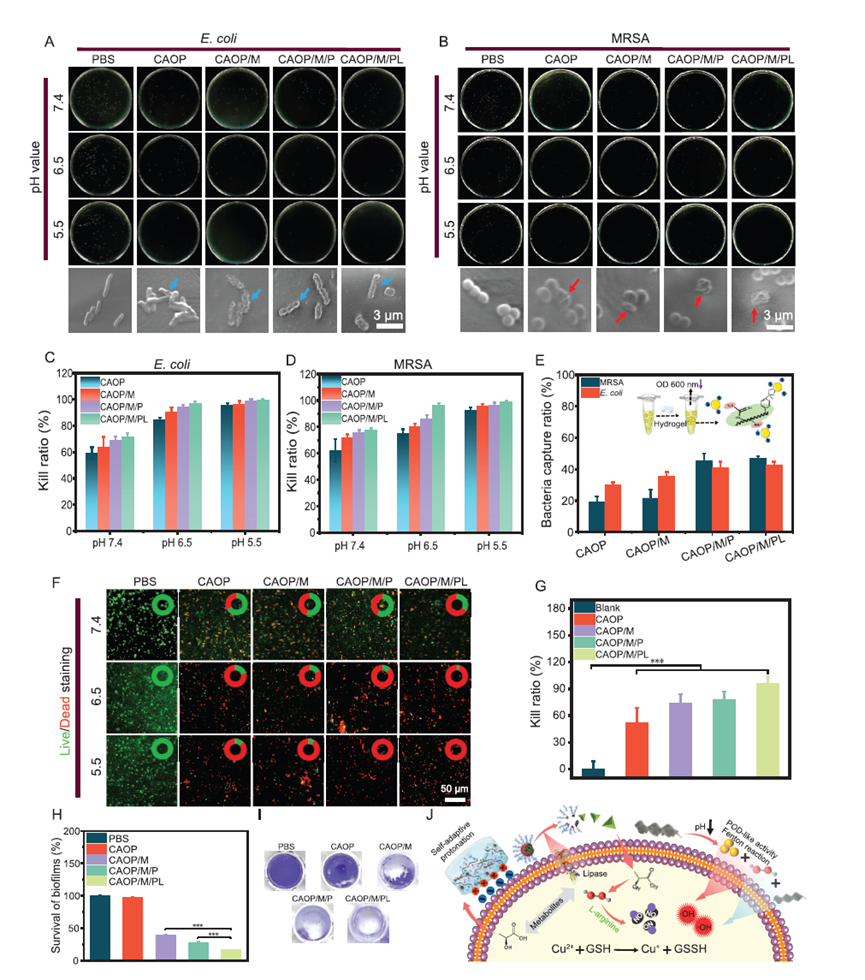
Figure 2: Illustration of the hydrogel’s antibacterial efficiency and biofilm removal capabilities against MRSA, as confirmed by live/dead staining and biofilm residue analysis.
Unlike static wound care dressings, this hydrogel continuously adapts to the changing environment of an infected wound. It transitions from delivering antibacterial treatment during active infection to promoting tissue regeneration as healing progresses. By dynamically responding to the acidic pH and enzymes released by bacteria, the hydrogel releases therapeutic agents, including reactive oxygen species (ROS) and nitric oxide (NO), which directly target biofilms and bacteria.
How It Works
The hydrogel is engineered with several sophisticated components that function in harmony:
- Materials Designed for Precision:
- The hydrogel incorporates L-arginine-modified chitosan (CA) and phenylboronic acid-modified oxidized dextran (ODP). These materials are held together by dynamic chemical bonds that enable self-healing properties, ensuring the hydrogel remains intact even when exposed to mechanical stress from motion wounds.
- Responsive Mechanisms:
- Bacterial infection lowers the pH of the wound environment. This triggers the hydrogel to collapse its structure, releasing encapsulated micelles and nanozymes. These elements include lactate oxidase, which catalyzes the breakdown of lactic acid into hydrogen peroxide (H₂O₂), a substance lethal to bacteria.
- Cascade Reactions:
- Hydrogen peroxide, once released, activates further chemical reactions involving nanozymes (such as MoS₂ nanosheets coated with copper oxide). These reactions produce ROS and NO, which penetrate biofilms and kill bacteria at the molecular level.
- NO also encourages the formation of new blood vessels (angiogenesis), a crucial process for wound repair, while reducing oxidative stress.
- Dual-Phase Healing:
- During the infection phase, the hydrogel delivers antibacterial agents to clear the infection. As the infection subsides, it transitions to releasing factors that enhance collagen deposition and vascular regeneration, helping to rebuild healthy tissue.
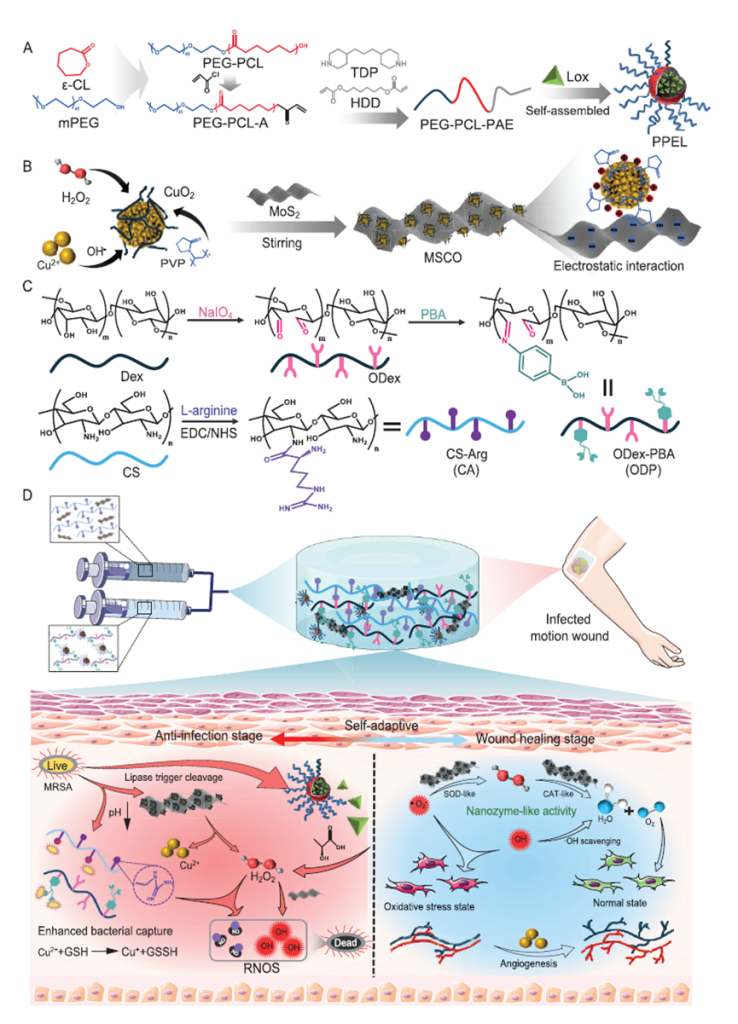
Figure 3: A schematic representation of the bacteria-responsive hydrogel mechanism, showcasing its dynamic response to bacterial metabolites, release of ROS and NO, and dual-phase function in infection eradication and tissue regeneration.
This design ensures that the hydrogel adapts to each stage of wound healing, addressing both infection and tissue repair needs.
Implications for Medicine
The potential of this hydrogel extends beyond wound care. Its applications could revolutionize surgical dressings, drug delivery systems, and infection control strategies. By minimizing reliance on traditional antibiotics, the hydrogel offers a sustainable solution to combatting drug resistance.
Furthermore, its self-healing and motion-adaptive properties make it particularly suitable for wounds in areas prone to movement, such as joints or the neck. This could reduce the frequency of dressing changes, improve patient comfort, and lower healthcare costs.
Conclusion
This bacteria-responsive hydrogel represents a significant step forward in the design of intelligent biomaterials. By addressing the dual challenges of bacterial infection and delayed healing, it provides a promising new option for clinicians. As the medical community seeks to tackle the growing problem of antibiotic resistance, innovations like this hydrogel highlight the importance of integrating material science with medicine.
The next phase of research will likely focus on clinical trials to assess the hydrogel’s safety and efficacy in human patients. If successful, it could set a new standard for personalized wound care, providing tailored solutions to some of the most persistent challenges in healthcare.
Reference
Yang Y., Wang J., Huang S., Li M., Chen J., Pei D., Tang Z., Guo B. Bacteria-responsive programmed self-activating antibacterial hydrogel to remodel regeneration microenvironment for infected wound healing. National Science Review. 2024;11:nwae044. https://doi.org/10.1093/nsr/nwae044.
