Editor: Sarah
Oral cancer, particularly oral squamous cell carcinoma (OSCC), remains a global healthcare challenge, ranking among the top 15 most common cancers worldwide. Its aggressive recurrence and metastatic nature make it particularly daunting to treat. While early detection provides an 80% five-year survival rate, this plummets to less than 20% for late-stage diagnoses. Conventional treatments—surgery, chemotherapy, and radiation therapy—while effective, often come at the cost of significant side effects, reduced quality of life, and high recurrence rates.
Amid these challenges, the development of nano-drug delivery systems (nano-DDSs) represents a pivotal advancement. These microscopic carriers enable targeted drug delivery, minimizing toxicity to healthy tissues while improving therapeutic outcomes.
Traditional Treatments: The Need for Innovation
Standard OSCC therapies, including surgery, radiation, and chemotherapy, often result in unintended damage to surrounding healthy tissues. Surgical procedures can impair vital functions like speaking and eating, while chemotherapy and radiation frequently cause side effects ranging from oral infections to systemic toxicity. The associated physical and emotional toll on patients underscores the urgent need for innovative treatment approaches.
Nano-DDSs address many of these challenges by leveraging nanocarriers like PLGA (poly(lactic-co-glycolic acid)) and PEG (polyethylene glycol). These materials enable precise drug delivery to tumor sites, enhancing drug accumulation and retention while reducing off-target effects.
Advances in Nano-DDS Technology
- PLGA-Based Nanoparticles: PLGA nanoparticles have demonstrated significant potential in improving cancer treatments. For instance, encapsulating the chemotherapy drug doxorubicin (DOX) within PLGA nanoparticles enhances drug stability and achieves sustained release. Studies reveal a 30% higher inhibition rate of SCC-15 oral cancer cells compared to traditional delivery methods.
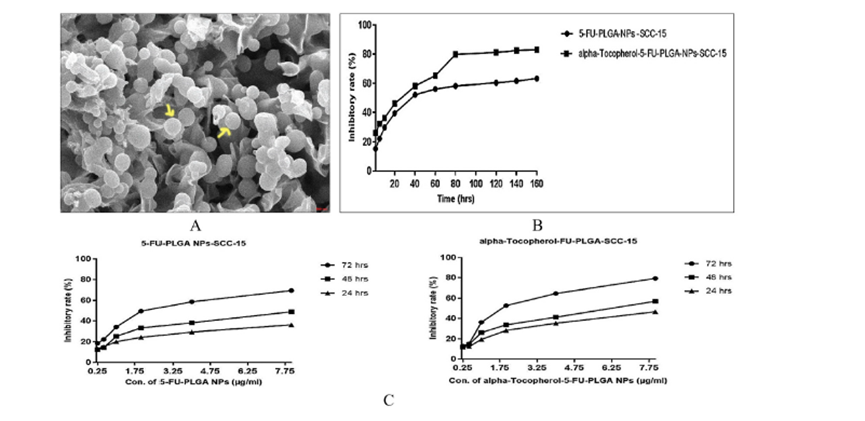
Figure 1: Scanning Electron Microscope Image and Drug Inhibition Rates
- Ligand-Modified Nanocarriers: By modifying nanoparticles with ligands such as folic acid or PD-L1-targeting antibodies, researchers have achieved greater precision in drug delivery. PD-L1-targeted nanoparticles have been shown to not only inhibit tumor growth in preclinical models but also activate immune cells near the tumor, aligning with the principles of immunotherapy.
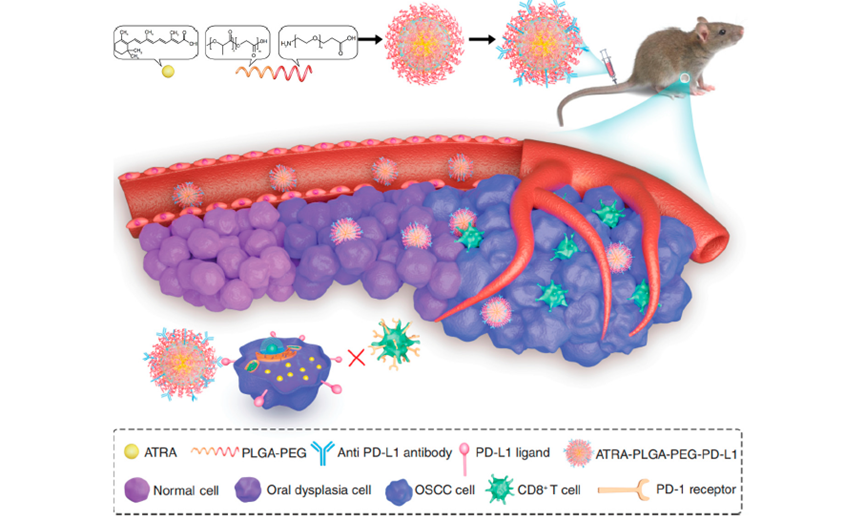
Figure2: Fabrication and Mechanism of ATRA-PLGA-PEG-PD-L1 Nanomedicine
The Promise of Nano-DDSs
Nano-DDSs offer multiple benefits:
- Enhanced Precision: Targeted delivery reduces damage to healthy tissues.
- Improved Therapeutic Outcomes: Controlled release minimizes toxicity and increases drug efficacy.
- Cost Efficiency: By reducing hospital stays and complications, nano-DDSs could lower overall healthcare costs.
An example includes PLGA nanoparticles functionalized with folic acid ligands, which demonstrated selective targeting of OSCC cells overexpressing folic acid receptors. Such approaches exemplify the potential for precision medicine in oral cancer treatment.
Challenges and Future Directions
Despite their promise, nano-DDSs face obstacles that need to be addressed before widespread clinical adoption:
- Scalability: Producing nanoparticles in large quantities while maintaining quality remains a technical challenge.
- Safety Assessments: Long-term studies on the toxicity and biodegradability of nanomaterials are essential.
- Regulatory Approval: Aligning with stringent safety and efficacy standards is a prerequisite for clinical use.
Future research should focus on optimizing nanoparticle designs, developing cost-effective manufacturing processes, and conducting robust clinical trials to establish safety and efficacy.
Conclusion
The progress in nano-DDSs highlights their potential to transform oral cancer treatment. By enhancing precision, reducing side effects, and aligning with precision medicine principles, nano-DDSs offer a promising path forward. While challenges remain, ongoing research inspires optimism for safer and more effective therapies that could significantly improve patient outcomes.
Reference
Zhang, Yun, et al. “Nano-Drug Delivery Systems in Oral Cancer Therapy: Recent Developments and Prospective.” Pharmaceutics, vol. 16, no. 1, 2024, p. 7, https://doi.org/10.3390/pharmaceutics16010007. Accessed 10 Jan. 2025.
