Editor: Nina
Research Question
- Challenge Addressed: How can a one-time gene editing therapy targeting hepatocytes help lower lipid levels and reduce cardiovascular disease risks?
Research Design and Strategy
- Focus on Hepatocyte-Specific Gene Editing: The study explored the feasibility of using a targeted base editing system specific to liver cells to inactivate ANGPTL3, a key gene in lipid metabolism.
- One-Time Intervention: Designed to be a permanent solution for lipid control, the strategy bypasses the limitations of existing therapies requiring continuous administration.
- Dual-AAV9 Delivery System: AAV9 was used to transport base editor components, ensuring safe and efficient liver-specific delivery.
- Hepatocyte-Specific Expression: The human alpha-1-antitrypsin (hAAT) promoter was employed to restrict gene editing to liver cells, minimizing off-target effects.
Method
- Cytosine Base Editor (CBE): AncBE4max, a cytosine base editor, was optimized to induce precise mutations in ANGPTL3, introducing a premature stop codon to eliminate its activity.
- Split Base Editor Delivery: Due to the size constraints of AAV, the base editor was split into two components (N-terminal and C-terminal halves) and reassembled inside hepatocytes using an intein-split strategy.
- Guide RNA (gRNA): A specific gRNA was designed to target the ANGPTL3 gene, enabling efficient editing.
- Mouse Model: Male C57BL/6J mice were treated with the dual-AAV9 system via tail vein injections, allowing systemic distribution to the liver.
Key Results
- Editing Efficiency: Achieved 63.3% gene editing efficiency in bulk liver tissue, introducing a loss-of-function mutation in ANGPTL3.
- Lipid Reductions: Serum triglycerides and total cholesterol levels were reduced by 58% and 61%, respectively, within four weeks.
- Protein Knockdown: ANGPTL3 protein levels in the bloodstream were nearly undetectable within two to four weeks post-treatment.
- Safety: The treatment showed no significant liver toxicity or off-target effects, as confirmed by histological and biochemical analyses.
Significance of the Research
- Permanent Therapeutic Option: Offers a single-administration therapy for lifelong lipid control, addressing a significant gap in cardiovascular disease management.
- Targeted and Safe: Demonstrates the feasibility of liver-specific base editing to achieve precise genetic modifications while minimizing risks to other tissues.
- Foundation for Future Research: Establishes a platform for exploring gene editing applications for other metabolic and genetic diseases.
Advances in Cardiovascular Treatments: The Role of Genetic Insights
Cardiovascular disease management has significantly evolved over the decades, but existing therapies often fall short in addressing severe hypercholesterolemia and treatment-resistant cases. While statins and PCSK9 inhibitors have proven effective, many patients fail to achieve optimal lipid control due to genetic factors or drug intolerance.
The study leverages advances in genetic research, focusing on the ANGPTL3 gene, which influences lipid metabolism. Loss-of-function variants of ANGPTL3 have been associated with lower lipid levels and reduced cardiovascular risks. Unlike conventional therapies requiring repeated administration, gene editing offers the promise of a single intervention with lasting effects.
By exploring hepatocyte-specific base editing, the study pioneers a targeted strategy for lipid regulation, bypassing limitations of existing treatments.
Research Focus: Precision Editing for Lipid Control
The research team, composed of scientists from institutions including Indiana University and the Ohio State University, aimed to establish a liver-specific base editing system targeting ANGPTL3. Published in 2023, the study explores how AAV-based delivery of cytosine base editors can achieve efficient, targeted gene modification in vivo.
The study’s primary objective was to assess whether precise editing of ANGPTL3 in hepatocytes could reduce circulating lipid levels, addressing a significant gap in cardiovascular disease treatment.
Experimental Procedures and Results
1. Designing and Validating the Base Editing System
- Key Step: Two overlapping guide RNAs (gRNAs) were tested to introduce a premature stop codon (Q135X) in the ANGPTL3 gene using AncBE4max.
- Result: The gRNA1-AncBE4max combination achieved an in vitro editing efficiency of 51.5%.
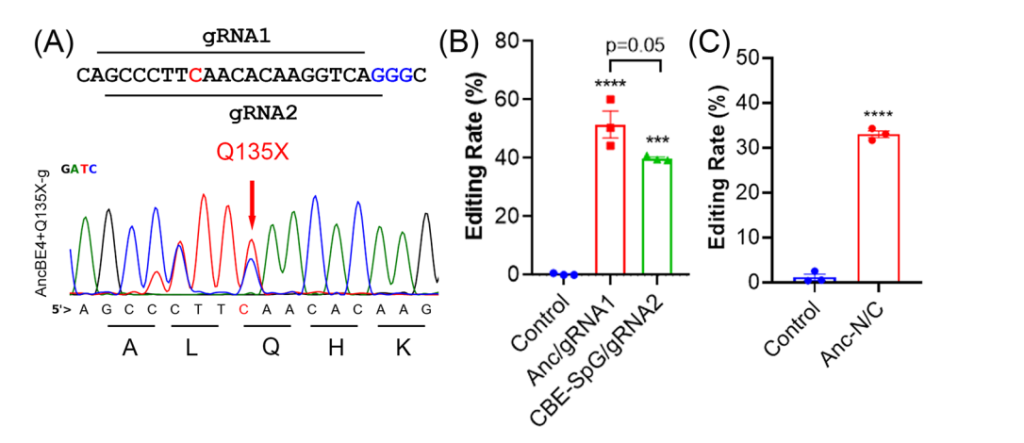
- Significance: Demonstrated the feasibility of targeting ANGPTL3 with base editors and validated the selection of gRNA1 for in vivo experiments.
- Key Innovation: Used a split-intein approach to package the base editor for delivery via AAV vectors, overcoming size constraints.
2. Systemic Delivery of Dual-AAV9 System in Mice
- Key Step: The dual-AAV9 system, containing the N- and C-terminal halves of the base editor and a liver-specific promoter, was administered via tail vein injection into eight male mice.
- Result: Editing efficiency of 63.3% was achieved in liver tissue, resulting in an 88% reduction in ANGPTL3 transcript levels and near-complete depletion of ANGPTL3 protein in the bloodstream within four weeks.
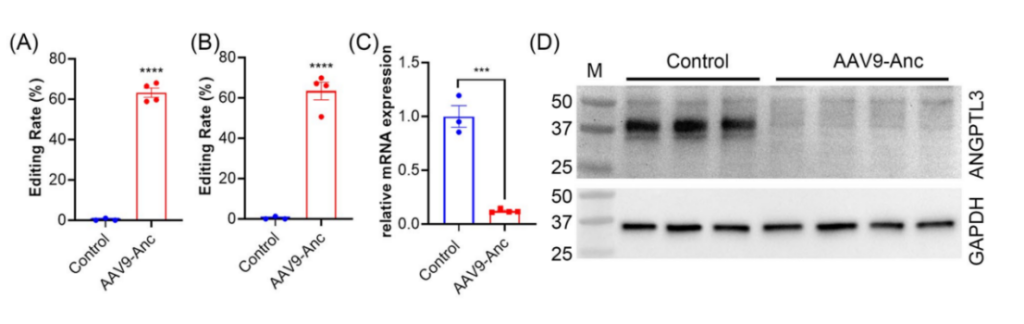
- Significance: Demonstrated the efficiency and specificity of the dual-AAV9 system in achieving liver-targeted base editing.
- Key Innovation: Hepatocyte specificity was achieved using the hAAT promoter, ensuring minimal off-target effects in non-liver tissues.
3. Reduction in Lipid Levels
- Key Step: Serum lipid levels were monitored weekly for four weeks post-treatment to assess the therapeutic impact of ANGPTL3 inactivation.
- Result: Triglyceride levels decreased from 19.3 mg/dL to 8.1 mg/dL (58% reduction), while total cholesterol levels dropped by 61%.

- Significance: Highlights the therapy’s potential to significantly lower lipid levels and reduce cardiovascular risks.
- Key Innovation: Demonstrated sustained lipid reductions with a one-time gene editing therapy.
4. Evaluation of Off-Target Effects
- Key Step: Off-target tissues (heart, kidney, and skeletal muscle) were analyzed for editing activity using Sanger sequencing and orthogonal R-loop assays.
- Result: No detectable off-target editing was observed in non-liver tissues.
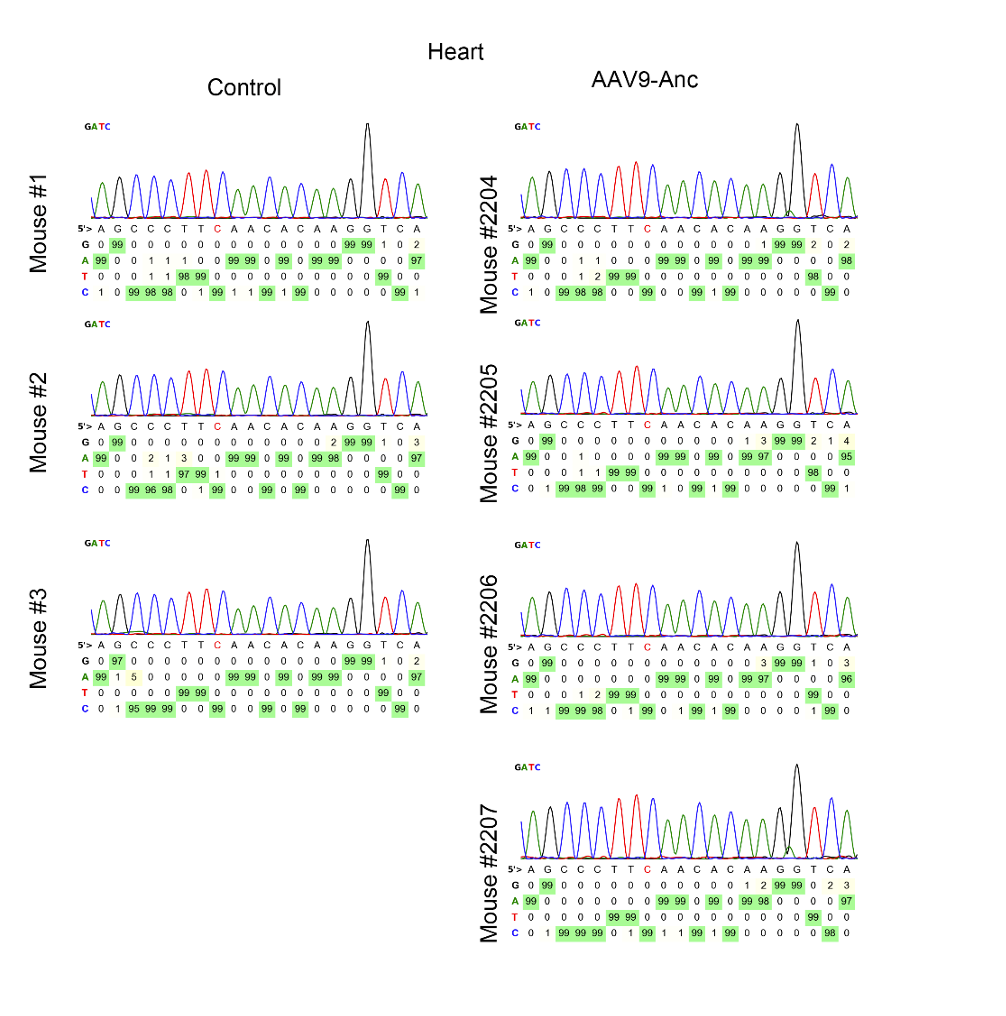
- Significance: Confirms the specificity and safety of the therapy for clinical applications.
- Key Innovation: Employed a highly specific delivery and expression system to minimize unintended editing.
5. Assessment of Safety and Liver Toxicity
- Key Step: Liver tissue was analyzed using histological methods and liver enzyme assays (ALT and AST) to evaluate potential toxicity.
- Result: No significant liver inflammation, T cell infiltration, or elevated liver enzymes were detected.
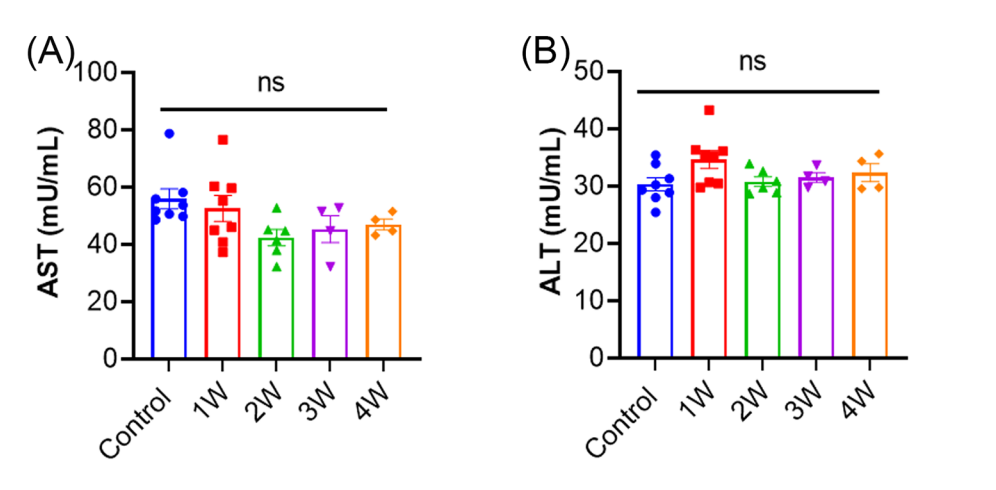
- Significance: Demonstrates the therapy’s safety profile and suitability for further development.
- Key Innovation: Integrated rigorous safety assessments into the experimental design to ensure clinical feasibility.
Conclusion: Towards Precision Cardiovascular Therapy
This study represents a promising advance in gene editing for cardiovascular disease management. By targeting ANGPTL3 with a hepatocyte-specific base editing system, researchers achieved significant reductions in lipid levels with minimal toxicity or off-target effects. While challenges such as transient delivery and off-target risks remain, the findings lay the groundwork for developing gene editing therapies as permanent solutions for hypercholesterolemia and related conditions.
Future studies will focus on optimizing delivery systems, such as lipid nanoparticles or viral-like particles, to ensure transient, safe, and scalable applications for clinical use. The success of this approach could revolutionize treatment paradigms for lipid disorders and metabolic diseases, offering patients a long-term solution to reduce cardiovascular risks.
