Key Preview: A Deep Dive into the Study’s Focus
- Key Questions Addressed: The study investigates the role of xCT-mediated ferroptosis in macrophages and its effect on tumor-associated macrophages (TAMs) and hepatocellular carcinoma (HCC). Can targeting this mechanism enhance the efficacy of anti-PD-L1 therapy?
- Methodological Insights: Researchers utilized genetic knockout models and advanced nanoparticle delivery systems to explore ferroptosis activation in macrophages. Techniques included RNA sequencing, immunofluorescence staining, and tumor progression assays.
- Significant Results: xCT knockout in macrophages suppressed tumor growth and metastasis, diminished TAM infiltration, and amplified ferroptosis. Nanoparticles delivering ferroptosis inducers combined with anti-PD-L1 therapy showed promising outcomes.
- Implications: Targeting macrophage-derived xCT can refine tumor microenvironment (TME) regulation, bolster immunotherapy, and guide future therapeutic strategies.
Introduction: Expanding Horizons in HCC Treatment
Hepatocellular carcinoma (HCC) remains one of the most fatal cancers globally. Research into the tumor microenvironment (TME), particularly the role of macrophages, has opened doors to innovative treatments. While immunotherapy has shown promise, its effectiveness is often hindered by protumoral macrophage polarization. This study highlights the dual potential of ferroptosis activation and immunotherapy, a significant leap in addressing these challenges.
Aiming for Precision: Research Scope and Significance
This groundbreaking study by Bufu Tang and collaborators, published in Advanced Science, underscores the therapeutic potential of xCT-targeted ferroptosis. By disrupting xCT expression in macrophages, the research aimed to diminish TAM-induced immunosuppression and enhance anti-PD-L1 therapy’s effectiveness.
Key Research Process: Decoding the Methodology and Results
1. Investigating the Role of xCT in Macrophages and Tumor Progression
The study began by investigating the role of xCT in macrophages using genetic knockout models in mice. Researchers generated wild-type (WT) and xCT knockout (KO) mice, specifically targeting the xCT gene in macrophages to examine its impact on HCC tumor progression. The results showed that tumors grew significantly slower in xCT KO mice compared to WT controls, with tumor volume and weight reduced by around 50%. Histological analysis confirmed lower proliferation markers and fewer blood vessels in xCT KO tumors. This suggested that xCT in macrophages plays a crucial role in promoting HCC growth and metastasis, highlighting its potential as a therapeutic target.
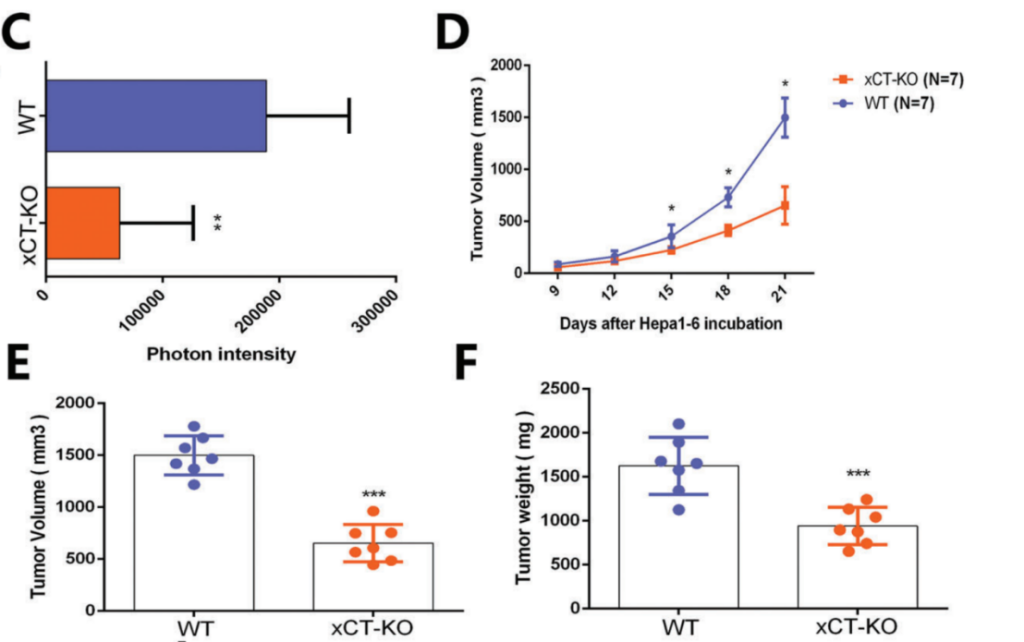
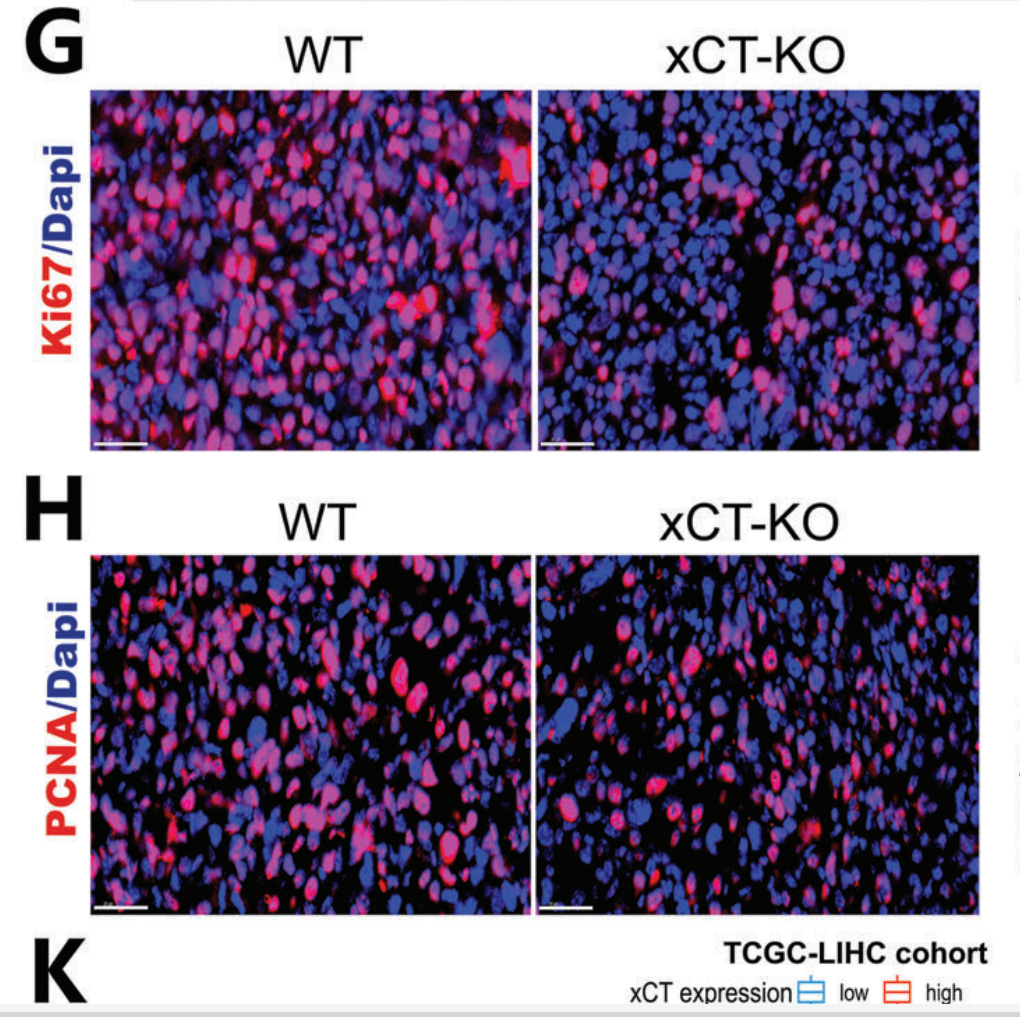
2. Effects of xCT Knockout on Macrophage Polarization and Ferroptosis
Next, the researchers assessed the effects of xCT knockout on macrophage polarization and ferroptosis activity. Tumor-associated macrophages (TAMs) were isolated from WT and xCT KO mice, and macrophage polarization was analyzed by flow cytometry and immunofluorescence. Results showed that xCT KO in TAMs significantly reduced the M2-like macrophage population, which is typically immunosuppressive, and promoted a shift toward M1-like polarization. Furthermore, ferroptosis markers, such as lipid peroxidation and iron accumulation, were elevated in xCT KO TAMs. This indicated that xCT is involved in protecting TAMs from ferroptotic cell death, and its deletion enhances ferroptosis, shifting the macrophage phenotype to a more antitumor state.
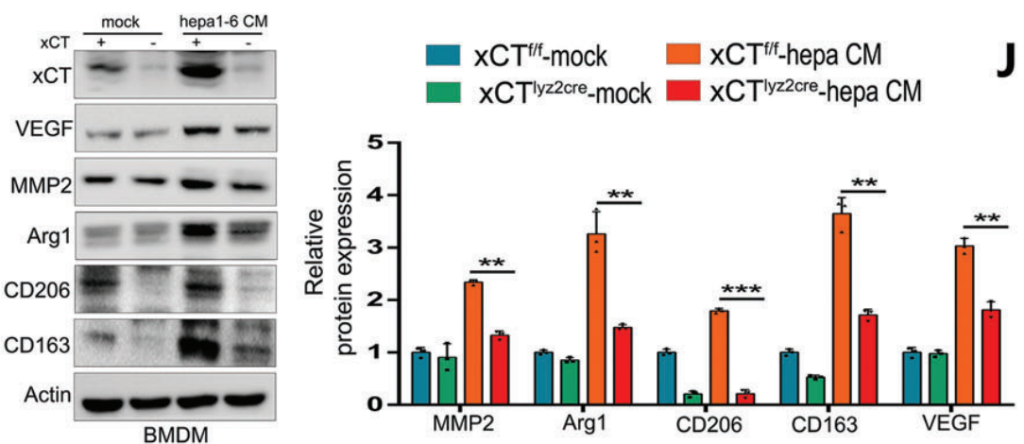
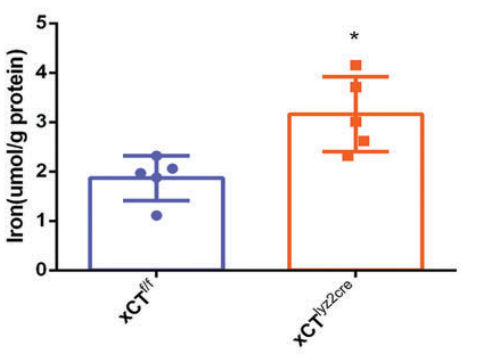
3. Targeted Ferroptosis Induction Using Man@pSiNPs Nanoparticles
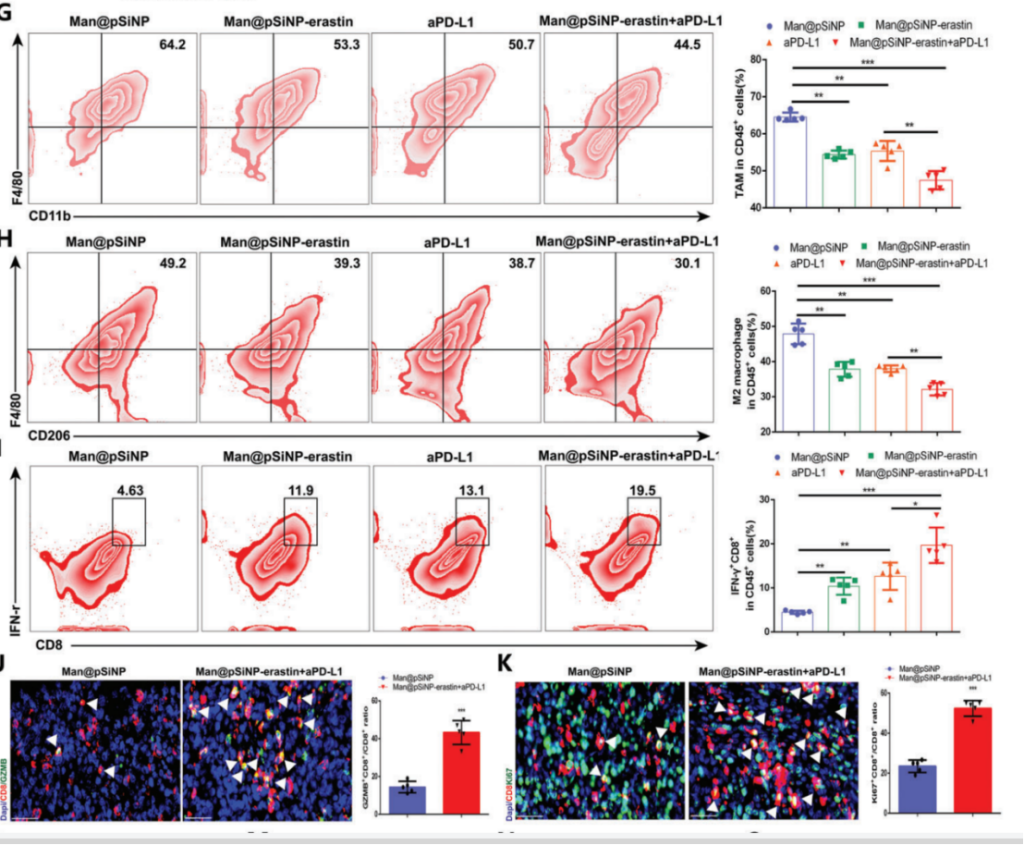
To target ferroptosis in TAMs, the team developed mannose-modified porous silicon nanoparticles (Man@pSiNPs) loaded with the ferroptosis inducer erastin. These nanoparticles were designed to specifically target TAMs, which overexpress the mannose receptor. In vivo experiments demonstrated that Man@pSiNPs successfully accumulated in TAMs, leading to significant ferroptosis induction. Tumor growth was substantially inhibited in the Man@pSiNPs-erastin treatment group, with a reduction in tumor volume compared to the control. The targeted delivery of ferroptosis inducers to TAMs also resulted in reduced M2 macrophage infiltration and enhanced immune cell activation, making this approach a promising strategy for enhancing tumor immunotherapy.
4. Combining Ferroptosis Inducers with Anti-PD-L1 Therapy for Enhanced Tumor Suppression
The final experiment tested the combination of Man@pSiNPs-erastin and anti-PD-L1 immunotherapy. Mice were treated with either Man@pSiNPs-erastin, anti-PD-L1, or both in combination. The results showed that the combined therapy significantly suppressed tumor growth, more so than either treatment alone. Tumor volume in the combination treatment group was reduced , and there was a noticeable increase in CD8+ T cell infiltration, a key marker of antitumor immunity. The combination therapy also led to a shift in TAM polarization from M2 to M1, enhancing the overall immune response. This experiment highlighted the synergistic potential of combining ferroptosis induction in TAMs with immune checkpoint blockade for more effective cancer therapy.
Key Innovations
Targeted Nanoparticle Delivery: The use of Man@pSiNPs to specifically deliver ferroptosis inducers to TAMs is a major innovation, ensuring targeted therapy with minimal off-target effects. This approach increases precision in modifying the TME and provides a novel method for enhancing immunotherapy efficacy.
Dual Strategy for TAM Reprogramming: By combining ferroptosis induction in TAMs with anti-PD-L1 therapy, the study creates a synergistic therapeutic approach that not only depletes immunosuppressive macrophages but also enhances immune checkpoint blockade, offering a promising avenue for overcoming resistance to immunotherapy.
Novel Mechanistic Insights: The study elucidates the role of xCT in regulating ferroptosis in TAMs, providing new insights into how targeting xCT can shift macrophage polarization and improve immunotherapy outcomes. This opens up potential therapeutic strategies for HCC that involve manipulating the TME and macrophage function.
These experiments demonstrate the potential of targeting xCT-mediated ferroptosis in TAMs as an effective strategy to enhance immunotherapy and suppress HCC progression, providing valuable insights into future cancer treatment strategies.
Conclusion: Implications and Future Directions
The findings highlight that macrophage ferroptosis, driven by xCT targeting, can reshape the TME to favor antitumor immunity. While the study demonstrates synergistic benefits of combining ferroptosis inducers with anti-PD-L1 therapy, it also calls for deeper exploration into clinical applications and safety evaluations.
Rference:
Tang, Bufu, et al. “Targeted xCT‐mediated ferroptosis and protumoral polarization of macrophages is effective against HCC and enhances the efficacy of the anti‐PD‐1/L1 response.” Advanced Science 10.2 (2023): 2203973.
